| Description |
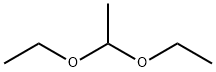
Acetal (full name: Acetaldehyde diethyl acetal/1,1-Diethoxyethane) is a major flavoring component of distilled beverages, especially malt whisky and sherry.
Acetaldehyde diethyl acetal is used as a flavoring agent to provide fruit, nut, rum, and whiskey flavors. It can react with diketene to form ethyl 5-ethoxy-3-oxohexanoate in the presence of titanium chloride. It can also be used to synthesize mixed acetal glycosides via transacetalation. |
| As a flavor ingredient |
Identification:
| CAS.No.: |
105-57-7 |
FL.No.: |
6.001 |
FEMA.No.: |
2002 |
NAS.No.: |
2002 |
| CoE.No.: |
35 |
EINECS.No.: |
203-310-6 |
JECFA.No.: |
941 |
|
|
|
| Synthesis |
Acetaldehyde diethyl acetal can be obtained by the reaction between ethyl alcohol and acetaldehyde in the presence of anhydrous calcium chloride. |
| References |
- Maarse, H. (1991). Volatile Compounds in Foods and Beverages. CRC Press. p. 553. ISBN 0-8247-8390-5.
- Zea, Luis; Serratosa, María P.; Mérida, Julieta; Moyano, Lourdes (2015). "Acetaldehyde as Key Compound for the Authenticity of Sherry Wines: A Study Covering 5 Decades". Comprehensive Reviews in Food Science and Food Safety. 14 (6): 681–693.
|
| Description |
Acetal is a clear, colourless, and extremely flammable liquid with an agreeable odour. The vapour is susceptible to cause flash fire. Acetal is sensitive to light and, on storage, may form peroxides. In fact, it has been reported to be susceptible to autoxidation and should, therefore, be classified as peroxidisable. Acetal is incompatible with strong oxidising agents and acids. |
| Chemical Properties |
clear, colorless liquid |
| Uses |
Solvent; in synthetic perfumes such as jasmine; in organic syntheses. |
| General Description |
A clear colorless liquid with a pleasant odor. Boiling point 103-104°C. Flash point -5°F. Density 0.831 g / cm3. Slightly soluble in water. Vapors heavier than air. Moderately toxic and narcotc in high concentrations. |
| Air & Water Reactions |
Highly flammable. Forms heat-sensitive explosive peroxides on contact with air. Slightly soluble in water. |
| Reactivity Profile |
Acetal can react vigorously with oxidizing agents. Stable in base but readily decomposed by dilute acids. Forms heat-sensitive explosive peroxides on contact with air. Old samples have been known to explode when heated due to peroxide formation [Sax, 9th ed., 1996, p. 5]. |
| Health Hazard |
May irritate the upper respiratory tract. High concentrations act as a central nervous system depressant. Symptoms of exposure include headache, dizziness, drowsiness, abdominal pain, and nausea. |
| Safety Profile |
Moderately toxic by ingestion, inhalation, and intraperitoneal routes.A skin and eye irritant. A narcotic. Dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Forms heat-sensitive explosive peroxides on contact with air. when heated to decomposition it emits acrid smoke and fumes. See also ETHERS and ALDEHYDES. |
| Purification Methods |
Dry acetal over Na to remove alcohols and H2O, and to polymerise aldehydes, then fractionally distil. Or, treat it with alkaline H2O2 at 40-45o to remove aldehydes, then saturate with NaCl, separate, dry with K2CO3 and distil it from Na [Vogel J Chem Soc 616 1948]. [Beilstein 1 IV 3103.] |