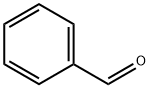
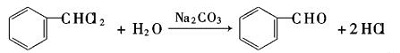Benzaldehyde
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1 g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameBenzaldehyde
- CAS No. 100-52-7
- EINECS No.202-860-4
- MFC7H6O
- MW106.12
- InChIKeyHUMNYLRZRPPJDN-UHFFFAOYSA-N
- AppearanceneatPale yellow
- Water Solubility <0.01 g/100 mL at 19.5 ºC
- Melting point -26 °C (lit.)
- Boiling point 178-179 °C (lit.)
- density 1.044 g/cm3 at 20 °C (lit.)
- storage temp. Store below +30°C.
AD68
| Benzaldehyde Basic information |
| Product Name: | Benzaldehyde |
| Synonyms: | almondartificialessentialoil;Artifical essential oil of almond;Artificial Almond Oil;Artificial bitter almond oil;artificialalmondoil;Benzaldehyde FFC;benzaldehydeffc;Benzene carbaldehyde |
| CAS: | 100-52-7 |
| MF: | C7H6O |
| MW: | 106.12 |
| EINECS: | 202-860-4 |
| Product Categories: | Pharmaceutical Intermediates;Benzaldehyde;A-F;A-B;Aldehydes;Alpha Sort;Analytical Standards;Analytical/Chromatography;Beverage Standards;Building Blocks;C7;Carbonyl Compounds;Chemical Synthesis;Chromatography;Environmental Standards;Flavors and Fragrances;Food &;Nutraceuticals;Organic Building Blocks;Volatiles/ Semivolatiles;Aspalathus linearis (Rooibos tea);Carthamus tinctorius (Safflower oil);Ephedra sinica;Nutrition Research;Ocimum basilicum (Basil);Panax ginseng;Phytochemicals by Plant (Food/Spice/Herb);Sambucus nigra (Elderberry);Vaccinium macrocarpon (Cranberry);Vaccinium myrtillus (Bilberry);Zingiber officinale (Ginger);Solvent |
| Mol File: | 100-52-7.mol |
 |
|
| Benzaldehyde Chemical Properties |
| Melting point | -26 °C |
| Boiling point | 179 °C |
| density | 1.044 g/cm 3 at 20 °C(lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | 4 mm Hg ( 45 °C) |
| refractive index | n20/D 1.545(lit.) |
| FEMA | 2127 | BENZALDEHYDE |
| Fp | 145 °F |
| storage temp. | room temp |
| solubility | H2O: soluble100mg/mL |
| pka | 14.90(at 25℃) |
| PH | 5.9 (1g/l, H2O) |
| explosive limit | 1.4-8.5%(V) |
| Water Solubility | <0.01 g/100 mL at 19.5 ºC |
| FreezingPoint | -56℃ |
| Sensitive | Air Sensitive |
| Merck | 14,1058 |
| BRN | 471223 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, reducing agents, steam. Air, light and moisture-sensitive. |
| InChIKey | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
| CAS DataBase Reference | 100-52-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde(100-52-7) |
| EPA Substance Registry System | Benzaldehyde(100-52-7) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 22 |
| Safety Statements | 24 |
| RIDADR | UN 1990 9/PG 3 |
| WGK Germany | 1 |
| RTECS | CU4375000 |
| F | 8 |
| TSCA | Yes |
| HazardClass | 9 |
| PackingGroup | III |
| Hazardous Substances Data | 100-52-7(Hazardous Substances Data) |
| Toxicity | LD50 in rats, guinea pigs (mg/kg): 1300, 1000 orally (Jenner) |
| MSDS Information |
| Provider | Language |
|---|---|
| Benzaldehyde | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Benzaldehyde Usage And Synthesis |
| Introductioin | Benzaldehyde(C6H5CHO) is an aromatic aldehyde bearing a single formyl group with an almond odor. Benzaldehyde occurs naturally in bitter almond oil, patchouli oil, hyacinth oil and other essential oils. It is widely used as a precursor to prepare various aniline dyes, perfumes, flavorings, and pharmaceuticals, and as a solvent, plasticizer and cryogenic lubricant used industrially. |
| description | Benzaldehyde is an organic compound, and is synthetized by the way that the hydrogen of benzene is substituted by aldehyde. It is the most simple, and also the most commonly used industrial aromatic aldehyde. It is a colorless liquid at room temperature and has a special almond odor. Benzaldehyde is a compound that aldehyde is directly linked to the phenyl group, because it has a similar bitter almond flavor. Benzaldehyde widely exists in plant, especially in the Rosaceae plants. It is mainly in the form of glycosides in plant stem bark, leaves or seeds, such as amygdalin, bitter almond, cherry, laurel, peach. Benzaldehyde is naturally in bitter almond oil, patchouli oil, hyacinth oil, cananga oil. The compound is also in the nutlets and nuts, and exists in the form of Amygdalin, which is combination of glycosides. The chemical properties of Benzaldehyde is similar to that of aliphatic aldehydes, but It is also different. Benzaldehyde cannot reduce fehling reagent. When the reducing fat is used to reduce the benzaldehyde, the main products are benzene methanol, four substituted for the ortho-glycol and two-phenyl ethylene glycol. In the presence of potassium cyanide, two molecules of benzaldehyde form benzoin by acceptance the hydrogen atom. The substitution reaction in aromatic nucleus of benzaldehyde is mainly the meta-position product. For example, the main product is the m-nitrobenzaldehyde , when benzaldehyde is nitrated. |
| Uses | 1. Benzaldehyde is an important raw material for medicine, dyestuff, perfume and resin industry. It also can be used as solvent, plasticizer and low temperature lubricant. In essence, it is mainly used for the deployment of food flavor. A small amount of benzaldehyde is daily use in flavor and flavor of tobacco. In spite of being widely used as commercial food condiment and industrial solvents, the main use of benzyl alcohol is still used to synthesize a variety of other compounds from pharmaceuticals to plastic additives. Benzyl alcohol is an important intermediate product in the production of perfumes, spices, and some aniline dyes. Mandelic acid was synthesized by benzaldehyde as the starting reagent: With the first hydrocyanic acid reacts with benzaldehyde, then mandelonitrile hydrolyzed to Racemic mandelic acid. Glacialist LaChepelle and Stillman reported Ice crystallization is inhibited by benzaldehyde and aldehydes ice in 1966, so as to prevent the thick frost formation (Depth Hoar). This process can prevent snowslide caused by the instability of the snow cover. However, this compound has not been used extensively, because of the destruction of vegetation and polluted water sources. 2.It is mainly used for the preparation of flavors, such as almond, cherry, peach, nuts, etc., the amount is up to 40%. As aromatizing agent canned cherry syrup, adding amount is sugar 3mL/kg. 3. Pharmaceutical, dyestuff, spice intermediates. For the production of oxygen based benzene formaldehyde, lauric acid, lauric aldehyde, malachite, benzyl benzoate, benzyl aniline and benzylidene acetone etc.. Used to tune the soap flavor, edible essence, etc. 4. As the head of the special aroma, it is used trace formula for fragrance, such as lilac, white, violet, jasmine, acacia, sunflower, sweet plum, orange flower, Tofu pudding etc.. Also it is used in soap. Also it can be used as edible spices for almond, coconut cream, berries, cherries, apricots, peaches, plums, walnuts, and vanilla bean, spicy flavor. Wine with flavors such as rum, brandy, etc. 5. Benzaldehyde is an intermediate of herbicide resistance, plant growth regulator, and anti-amine. 6. Used as a reagent for the determination of ozone, phenol, alkaloid and methylene. Used in the preparation of spices. |
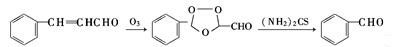
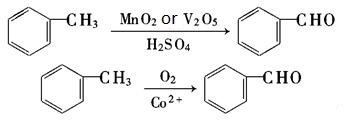
| Production | Benzaldehyde can be prepared by a variety of ways.
|
| reactions | Benzaldehyde can be slowly oxidized to benzoic acid in air, so a small amount of hydroquinone is often added to prevent its oxidation.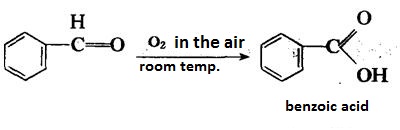 There is no α-H atom in the benzaldehyde molecule. Disproportionation reaction(Cannizarro reaction) may occur under the action of concentrated alkali: 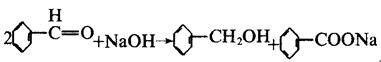 Heating benzaldehyde in the presence of catalyst of cyanide ion, it will occur bimolecular condensation: 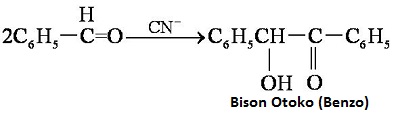 |
| Chemical Properties | Colorless to light yellow liquid |
| Uses | Manufacture of dyes, perfumery, cinnamic and mandelic acids, as solvent; in flavors. |
| General Description | A clear colorless to yellow liquid with a bitter almond odor. Flash point near 145°F. More denser than water and insoluble in water. Hence sinks in water. Vapors are heavier than air. The primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways. Used in flavoring and perfume making. |
| Air & Water Reactions | Oxidizes in air to form benzoic acid, which is moderately toxic by ingestion. Insoluble in water. |
| Reactivity Profile | A nontoxic, combustible liquid, reacts with oxidizing reagents. Benzaldehyde must be blanketed with an inert gas at all times since Benzaldehyde is oxidized readily by air to benzoic acid [Kirk-Othmer, 3rd ed., Vol. 3, 1978, p. 736]. In contact with strong acids or bases Benzaldehyde will undergo an exothermic condensation reaction [Sax, 9th ed., 1996, p. 327]. A violent reaction was observed on contact with peroxyacids (peroxyformic acid) [DiAns, J. et al., Ber., 1915, 48, p. 1136]. An explosion occurred when pyrrolidine, Benzaldehyde, and propionic acid were heated to form porphyrins. |
| Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Safety Profile | Poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. An allergen. Acts as a feeble local anesthetic. Local contact may cause contact dermatitis. Causes central nervous system depression in small doses and convulsions in larger doses. A skin irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible liquid. To fight fire, use water (may be used as a blanket), alcohol, foam, dry chemical. A strong reducing agent. Reacts violently with peroxyformic acid and other oxidizers. See also ALDEHYDES. |
| Purification Methods | To diminish its rate of oxidation, benzaldehyde usually contains additives such as hydroquinone or catechol. It can be purified via its bisulfite addition compound but usually distillation (under nitrogen at reduced pressure) is sufficient. Prior to distillation it is washed with NaOH or 10% Na2CO3 (until no more CO2 is evolved), then with saturated Na2SO3 and H2O, followed by drying with CaSO4, MgSO4 or CaCl2. [Beilstein 7 IV 505.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- BENZALDEHYDE 99%
- Inquiry
- 2024-11-05
-
VIP1年
- SS Reagents and Chemicals
- 100-52-7 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- Benzaldehyde 98.5% Extrapure 100-52-7 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Panorama Aromatics Ltd
- 100-52-7 Benzaldehyde 98%
- Inquiry
- 2024-03-29
-
VIP1年
- Shimmer Chemicals Pvt Ltd
- 100-52-7 99%
- Inquiry
- 2024-03-02
-
VIP1年
- Orgamine Chemicals(I) Pvt Ltd
- 100-52-7 Benzaldehyde 98%
- Inquiry
- 2024-03-01
-
VIP1年
- Aamirav Ingredients And Specialties Pvt Ltd
- Benzaldehyde 100-52-7 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Gujarat Alkalies and Chemicals Ltd
- Benzaldehyde 98%
- Inquiry
- 2024-02-21
-
VIP1年
- Sanghvi Organics Pvt Ltd
- 100-52-7 99%
- Inquiry
- 2024-02-15
-
VIP1年
- Prakash Chemicals Agencies
- 100-52-7 Benzaldehyde 99%
- Inquiry
- 2024-02-14
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY