

1-Nonanal
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1 g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name1-Nonanal
- CAS No.124-19-6
- EINECS No.204-688-5
- MFC9H18O
- MW142.24
- InChIKeyGYHFUZHODSMOHU-UHFFFAOYSA-N
- AppearanceLiquidClear colorless to light yellow
- Water Solubility Practically insoluble
- storage temp. Store below +30°C.
- density 0.827 g/mL at 25 °C (lit.)
- Boiling point 93 °C/23 mmHg (lit.)
- Melting point -18°C
AD68
| 1-Nonanal Basic information |
| Product Name: | 1-Nonanal |
| Synonyms: | FEMA 2782;ALDEHYDE C-9;1-NONANAL;PELARGONIC ALDEHYDE;PELARGONALDEHYDE;N-NONYLALDEHYDE;NONANAL;NONANALDEHYDE |
| CAS: | 124-19-6 |
| MF: | C9H18O |
| MW: | 142.24 |
| EINECS: | 204-688-5 |
| Product Categories: | NJ - NZ;Volatiles/ Semivolatiles;Aldehydes;Alpha Sort;Alphabetic;Analytical Standards;Analytical/Chromatography;Building Blocks;C9;Carbonyl Compounds;Chemical Synthesis;Chromatography;Environmental Standards;N;N-O;Organic Building Blocks;Aspalathus linearis (Rooibos tea);Carthamus tinctorius (Safflower oil);Citrus aurantium (Seville orange);Nutrition Research;Ocimum basilicum (Basil);Phytochemicals by Plant (Food/Spice/Herb);Vaccinium myrtillus (Bilberry);Zingiber officinale (Ginger) |
| Mol File: | 124-19-6.mol |
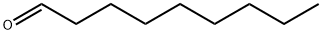 |
|
| 1-Nonanal Chemical Properties |
| Melting point | -18°C |
| Boiling point | 93 °C23 mm Hg(lit.) |
| density | 0.827 g/mL at 25 °C(lit.) |
| vapor pressure | ~0.26 mm Hg ( 25 °C) |
| refractive index | n20/D 1.424(lit.) |
| FEMA | 2782 | NONANAL |
| Fp | 147 °F |
| storage temp. | 2-8°C |
| form | Liquid |
| color | Clear colorless to light yellow |
| Water Solubility | Practically insoluble |
| BRN | 1236701 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 124-19-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Nonanal(124-19-6) |
| EPA Substance Registry System | Nonanal(124-19-6) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39 |
| RIDADR | 3082 |
| WGK Germany | 2 |
| RTECS | RA5700000 |
| TSCA | Yes |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29121900 |
| Hazardous Substances Data | 124-19-6(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Nonyl aldehyde | English |
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| 1-Nonanal Usage And Synthesis |
| Chemical Properties | brown liquid |
| Uses | A component of essential oils, Nonanal possesses a strong fruity odor. Essential oils have varying effects from antibacterial activity to hypolipidemic activity. |
| Definition | ChEBI: A fatty aldehyde formally arising from reduction of nonanoic acis. Metabolite observed in cancer metabolism. |
| General Description | Clear brown liquid characterized by a rose-orange odor. Insoluble in water. Found in at least 20 essential oils, including rose and citrus oils and several species of pine oil. |
| Air & Water Reactions | Sensitive to air. Insoluble in water. |
| Reactivity Profile | 1-Nonanal is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Polymerizes readily with sulfuric acid and oxidized to nonanoic acid. |
| Fire Hazard | 1-Nonanal is combustible. |
| Safety Profile | A severe skin irritant. Combustible liquid. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- HJ AROCHEM
- 124-19-6 99%
- Inquiry
- 2023-12-11
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
