

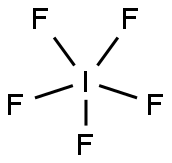
Pentalfluoroiodide
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1 g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NamePentalfluoroiodide
- CAS No.7783-66-6
- EINECS No.232-019-7
- MFF5I
- MW221.9
- Appearanceyellow liquidyellow
- density 3,2 g/cm3
- Boiling point 104.5°C
- Melting point 9.4°C
- Water Solubility reacts violently with H2O [HAW93]
AD68
| Pentalfluoroiodide Basic information |
| Product Name: | Pentalfluoroiodide |
| Synonyms: | IF5;Iodine fluoride;Iodine fluoride (IF5);Iodinefluoride;iodinefluoride(if5);Pentalfluoroiodide;Pentafluoroiodine;IODINE PENTAFLUORIDE |
| CAS: | 7783-66-6 |
| MF: | F5I |
| MW: | 221.9 |
| EINECS: | 232-019-7 |
| Product Categories: | Inorganic Fluorides |
| Mol File: | 7783-66-6.mol |
 |
|
| Pentalfluoroiodide Chemical Properties |
| Melting point | 9.4°C |
| Boiling point | 104.5°C |
| density | 3,2 g/cm3 |
| form | yellow liquid |
| CAS DataBase Reference | 7783-66-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Iodine pentafluoride(7783-66-6) |
| EPA Substance Registry System | Iodine fluoride (IF5)(7783-66-6) |
| Safety Information |
| Hazard Codes | O |
| Risk Statements | 8-14-34 |
| Safety Statements | 7/9-17-36/37/39-45 |
| RIDADR | 2495 |
| Hazard Note | Oxidising agent |
| HazardClass | 5.1 |
| PackingGroup | I |
| Pentalfluoroiodide Usage And Synthesis |
| Chemical Properties | Fuming liquid.Attacks glass. |
| Uses | Mild fluorinating agent. |
| General Description | A toxic colorless fuming liquid (m.p. 9° C). Decomposed by water to iodine and hydrofluoric acid. Contact with organic materials may cause their ignition. Corrosive to metals and tissue. Prolonged exposure of the container to fire or heat may result in their violent rupturing and rocketing. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. |
| Air & Water Reactions | Fumes in air. Reaction with water or water-containing materials is violent, [Mellor 2, Supp. 1:176, 1956]. |
| Reactivity Profile | A powerful oxidizer. Attack glass. Reacts violently with water or strong bases (potassium hydroxide, sodium hydroxide). Pentalfluoroiodide chars and usually ignites organic matter. Contact with boron, silicon, red phosphorus, sulfur, arsenic, antimony, bismuth, molybdenum and tungsten causes incandescence. Contact with potassium or sodium leads to explosions. Causes aluminum (foil, powder) to ignite. Explosive reactions with tetraiodoethylene, diethylaminotrimethylsilane. Violent reactions wilh benzene, dimethyl sulfoxide, tetraiodoethylene [Bretherick, 5th ed., 1995, p. 1434]. IF5 reacts explosively with diethylaminotrimethylsilane even at low temperature. (Oates, G. et al., J. Chem. Soc., Dalton Trans., 1974, 1383). |
| Hazard | Dangerous fire risk, reacts violently with water. Toxic by ingestion and inhalation, corrosive to skin and mucous membranes. |
| Health Hazard | TOXIC; inhalation or contact with vapor, substance, or decomposition products may cause severe injury or death. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. |
| Fire Hazard | May ignite combustibles (wood, paper, oil, clothing, etc.). React vigorously and/or explosively with water. Produce toxic and/or corrosive substances on contact with water. Flammable/toxic gases may accumulate in tanks and hopper cars. Some may produce flammable hydrogen gas upon contact with metals. Containers may explode when heated. Runoff may create fire or explosion hazard. |
| Purification Methods | Rogers et al. [J Am Chem Soc 76 4843 1954] removed dissolved iodine from IF5 by agitating with a mixture of dry air and ClF3 in a Fluorothene beaker using a magnetic stirrer. The mixture is transferred to a still, and the more volatile impurities are pumped off as the pressure is reduced below 40mm. The still is gradually heated (kept at 40mm) to remove the ClF3 before IF5 distilled. Stevens [J Org Chem 26 3451 1961] pumped IF5 under vacuum from its cylinder, trapping it at -78o, then allowing it to melt in a stream of dry N2. HARMFUL VAPOURS. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 159-160 1963.] |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
