

Sulfur hexafluoride
| Price | USD1.00 |
| Packge | 1kg |
- Min. Order:1 g
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameSulfur hexafluoride
- CAS No.2551-62-4
- EINECS No.219-854-2
- MFF6S
- MW146.05
- Appearancecolorless gascolorless
- Water Solubility slightly
- density 6.602
- Boiling point −64 °C1 mm Hg(lit.)
- Melting point −50 °C(lit.)
AD68
| Sulfur hexafluoride Basic information |
| Product Name: | Sulfur hexafluoride |
| Synonyms: | (OC-6-11)-Sulfurfluoride;(oc-6-11)-sulfurfluoride(sf6;Elegas;Esaflon;Hexafluorure de soufre;hexafluoruredesoufre;hexafluoruredesoufre(french);OC-6-11 |
| CAS: | 2551-62-4 |
| MF: | F6S |
| MW: | 146.06 |
| EINECS: | 219-854-2 |
| Product Categories: | Inorganics;Inorganic Fluorides;Vitamin Ingredients;Chemical Synthesis;Compressed and Liquefied Gases;Synthetic Reagents |
| Mol File: | 2551-62-4.mol |
 |
|
| Sulfur hexafluoride Chemical Properties |
| Melting point | −50 °C(lit.) |
| Boiling point | −64 °C1 mm Hg(lit.) |
| density | 6.602 |
| vapor density | 5.11 (vs air) |
| vapor pressure | 22 mm Hg ( 21.1 °C) |
| form | colorless gas |
| Water Solubility | slightly |
| Merck | 13,9063 |
| Stability: | Stable. Non-flammable. Explodes on contact with disilane. Reacts with sodium. |
| CAS DataBase Reference | 2551-62-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfur hexafluoride(2551-62-4) |
| EPA Substance Registry System | Sulfur fluoride (SF6), (OC-6-11)-(2551-62-4) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 37 |
| Safety Statements | 38 |
| RIDADR | UN 1080 2.2 |
| WGK Germany | - |
| RTECS | WS4900000 |
| Hazard Note | Irritant |
| HazardClass | 2.2 |
| Hazardous Substances Data | 2551-62-4(Hazardous Substances Data) |
| MSDS Information |
| Provider | Language |
|---|---|
| Sulfur hexafluoride | English |
| SigmaAldrich | English |
| Sulfur hexafluoride Usage And Synthesis |
| Chemical Properties | Chemical properties of sulfur hexafluoride are very stable. And compared to selenium hexafluoride, the hydrolysis rate of sulfur hexafluoride is extremely low, this is due to the small radius sulfur atom, which resulting in six fluorine atoms form a larger steric hindrance around. However, the fluorine atom radius is not big, so the repulsive force between the six fluorine atoms is not too large, S-F bond is not easy to dissociate. Enthalpy of formation of sulfur hexafluoride is-1220 kJ/mol, but enthalpy of formation of sulfur hexafluoride is-74 kJ/mol. Thus, the radius of fluorine atom and sulfur atom radius cause the very stable of sulfur hexafluoride molecule together---the molecules themselves are difficult to disconnect bond and break down and the attack group is difficult to approach to the central atom, in the thermodynamics and kinetics, they are both stable. Studies have said sulfur hexafluoride can be stably present in the atmosphere for thousands of years. | |||||||||||||||||||||||||||||||||||||
| Molecular Structure | In VSEPR theory, sulfur hexafluoride molecular configuration is regular octahedron, which shows in Figure 1, the yellow is sulfur atom, the blue is fluorine atom. The hybrid orbital type of sulfur atom in sulfur hexafluoride is generally considered sp3d2. The angles between adjacent S-F bond are all 90 °. S-F bond length is 1.55 Å. The dipole moment is 0, and therefore they are non-polar molecules. 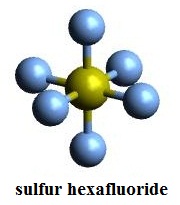 Figure 1 is a sulfur hexafluoride stick model. |
|||||||||||||||||||||||||||||||||||||
| Uses |
|
|||||||||||||||||||||||||||||||||||||
| Impact on the environment | Since the high intensity of infrared absorption, sulfur hexafluoride has been identified as a greenhouse gas, and it is the strongest greenhouse gases. 《Kyoto Protocol》indicate human must make great efforts to reduce the emissions of sulfur hexafluoride. Although sulfur hexafluoride in the atmosphere is very stable, when it once dissociate, it will generate S2F10, SF4 and HF and other fluorine-containing molecules, and these are corrosive and toxic substances, some of which are also greenhouse gases. | |||||||||||||||||||||||||||||||||||||
| Acute intravenous toxicity | Rabbit LD50: 5790 mg/kg | |||||||||||||||||||||||||||||||||||||
| Storage characteristics | Treasury ventilation low-temperature drying; Handle gently. | |||||||||||||||||||||||||||||||||||||
| Chemical Properties | Colorless gas; odorless. Slightly soluble in water; soluble in alcohol and ether. Noncombustible. | |||||||||||||||||||||||||||||||||||||
| Uses | In electrical circuit interrupters. In electronic ultra-high frequency piping. | |||||||||||||||||||||||||||||||||||||
| Brand name | SonoVue (for the microbubble formulation) (Ausimont). | |||||||||||||||||||||||||||||||||||||
| Reactivity Profile | This substance undergoes chemical reactions only under relatively severe circumstances. They are resistant to ignition, although they may become flammable at very high temperatures. They may be resistant to oxidation reduction, except in the most severe conditions. These materials may be nontoxic. They can asphyxiate. Contact of very cold liquefied gas with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container [Handling Chemicals Safely 1980]. | |||||||||||||||||||||||||||||||||||||
| Hazard | Asphyxiant. | |||||||||||||||||||||||||||||||||||||
| Health Hazard | Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases. | |||||||||||||||||||||||||||||||||||||
| Fire Hazard | Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Maharashtra Gas Co
- 2551-62-4 / 29267-82-1 Sulphur Hexafluoride 98%
- Inquiry
- 2024-01-19
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
