

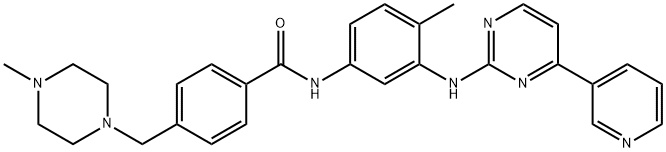
Imatinib
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product Name Imatinib
- CAS No.152459-95-5
- EINECS No.604-855-6
- MFC29H31N7O
- MW493.6
- AppearanceSolidWhite to Pale Beige
- Melting point 208-210°C (dec.)
- storage temp. Keep in dark place,Sealed in dry,Store in freezer, under -20°C
- density 1?+-.0.06 g/cm3(Predicted)
AD68
| Imatinib Basic information |
| Product Name: | Imatinib |
| Synonyms: | 4-(4-METHYL-PIPERAZIN-1-YLMETHYL)-N-[4-METHYL-3-(4-PYRIDIN-3-YL-PYRIMIDIN-2-YLAMINO)-PHENYL]-BENZAMIDE;Imatinib (4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide;Imatinib free base;Veenat;IMatinib(STI571);Imantinib base;4-(4-Methyl-piperazin-1-ylMethyl)-N-[4-Methyl-3-(4-pyridin-3-yl-pyriMidin-2-ylaM;IMatinib-D4 |
| CAS: | 152459-95-5 |
| MF: | C29H31N7O |
| MW: | 493.6 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Pharmaceutical intermediate;Imatinib;Aromatics;Heterocycles;Impurities;Intermediates & Fine Chemicals;Pharmaceuticals;API;GLEVEEC;Antineoplastic;Anti-cancer & immunity;Inhibitors;Molecular Targeted Antineoplastic |
| Mol File: | 152459-95-5.mol |
 |
|
| Imatinib Chemical Properties |
| Melting point | 208-210°C (dec.) |
| storage temp. | Refrigerator |
| pka | pKa1 8.07; pKa2 3.73; pKa3 2.56; pKa4 1.52(at 25℃) |
| Merck | 14,4902 |
| CAS DataBase Reference | 152459-95-5(CAS DataBase Reference) |
| Safety Information |
| Safety Statements | 24/25 |
| RTECS | CV5585673 |
| Imatinib Usage And Synthesis |
| Product description | Imatinib is a kind of oral drugs used for the treatment of the chronic myelogenous leukemia (abbreviated CML) of positive symptoms of Philadelphia chromosome (Ber-Abl). It is suitable for being applied to adult patients in acute transformation phase, accelerated phase, and chronic phase with treatment failure with interferon. CML is a kind of hematopoietic stem cell disease caused by the DNA abnormalities in the bone marrow stem cells. DNA abnormalities will produce abnormal proteins and interfere with the normal generation process of the white blood cells in the bone marrow, resulting in the sharp increase in the number of white blood cells. CML is divided into three phases including chronic phase, accelerated phase and crisis phase with the average survival period of the patients in crisis phase being only 2-3 months. Imatinib is also effective in treating the gastrointestinal stromal tumor with the efficiency being about 50%. |
| Approved indications in Europe, the United States and other countries | Novartis's imatinib (imatinib, Glivec) had been approved in Switzerland for being used as first-line drug for the treatment of early-stage adult chronic myelogenous leukemia and can also be applied to patients with various types of chronic granulocytic leukemia. Switzerland is the first countries which had approved to additionally include the above two indications of this drug. On July 28, 2006, the European Agency for the Evaluation of Medicinal Products (EMEA) had recommended the imatinib (Gleevec) of the Novartis Company for the treatment of two new indications-dermatofibrosarcoma protuberans (DFSP) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL). These final approvals of these two indications still need to wait the decision of the European Food and Drug Administration. In addition, Novartis has announced that the application of imatinib in treating hypereosinophilic syndrome and systemic mastocytosis is still in the approval process of FDA and EMEA. The drug has currently been approved in Europe, the United States and other countries for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia (Ph + CML) and gastrointestinal stromal tumors. The above information is edited by the Chemicalbook of Dai Xiongfeng. |
| Precautions | When combined with Ketoconazole, itraconazole, erythromycin, clarithromycin, the Cmax of this drug can (the maximum plasma concentration after the first drug administration) be increased by an average of 26% with the AUC being increased by 40%. The inducers of the metabolizing enzyme of hepatic drugs such as dexamethasone, phenytoin, carbamazepine, rifampicin and barbiturates can significantly reduce blood concentration of the drug. When the CYP3A4 metabolized substrates such as simvastatin, cyclosporine, pimozide, etc. were used in combined with imatinib, the plasma concentration of those drugs can be increased due to its competing with the drug enzyme. |
| Uses | Imatinib can be used for the treatment of the chronic myelogenous leukemia (abbreviated CML) of positive symptoms of Philadelphia chromosome (Ber-Abl). It is suitable for being applied to adult patients in acute transformation phase, accelerated phase, and chronic phase with treatment failure with interferon. |
| Chemical Properties | Orange Solid |
| Uses | Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & Imatinib |
| Uses | antineoplastic |
| Uses | atypical antipsychotic |
| Uses | Imatinib impurity. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- IMATINIB MESYLATE 152459-95-5 95-99%
- Inquiry
- 2024-09-11
-
VIP1年
- Medec Dragon Private Limited
- 152459-95-5 Imatinib 99%
- Inquiry
- 2024-03-19
-
VIP1年
- Sibram Pharmaceutical
- Imatinib 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Myosynth Laboratories Pvt Ltd
- Imatinib 152459-95-5 98%
- Inquiry
- 2024-03-14
-
VIP0年
- Glyra Health Care Pvt Ltd
- 152459-95-5 Imatinib 99%
- Inquiry
- 2024-02-21
-
VIP1年
- Alfa Omega Pharma
- Imatinib 152459-95-5 98%
- Inquiry
- 2024-02-17
-
VIP1年
- Sakar Healthcare
- Imatinib 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Ralington Pharma
- 152459-95-5 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Laurus Labs Ltd
- 152459-95-5 Imatinib 98%
- Inquiry
- 2024-02-02
-
VIP1年
- Kekule Pharma Limited
- Imatinib 152459-95-5 98%
- Inquiry
- 2024-01-31
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
