

Topiramate
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameTopiramate
- CAS No. 97240-79-4
- EINECS No.619-263-3
- MFC12H21NO8S
- MW339.36
- Appearancesolidwhite
- Melting point 125°C
- storage temp. 2-8°C
- Boiling point 438.7±55.0 °C(Predicted)
- density 1.336±0.06 g/cm3(Predicted)
- Water Solubility 9.705g/L(temperature not stated)
AD68
| Topiramate Basic information |
| Product Name: | Topiramate |
| Synonyms: | 2,3:4,5-bis-o-(1-methylethylidene)-beta-d-fructopyranossulfamate;MCN 4853;2,3:4,5-BIS-O-(1-METHYLETHYLIDENE)-36-D-FRUCTO-PYRANOSE SULFAMATE;2,3:4,5-BIS-O-(1-METHYLETHYLIDENE)-B-D-FUCTO-PYRANOSE SULFAMATE;2,3:4,5-bis-o-(1-methylethylidene)-beta-d-fructopyranose sulfamate;2,3:4,5-BIS-O-(1-METHYLETHYLIDENE)-BETA-D-FUCTOPYRANOSE SULFAMATE;RWJ 17021;RWJ-17021-000 |
| CAS: | 97240-79-4 |
| MF: | C12H21NO8S |
| MW: | 339.36 |
| EINECS: | 200-659-6 |
| Product Categories: | Active Pharmaceutical Ingredients;APIs;Health & Beauty;Carbohydrates & Derivatives;Pharmaceuticals;Glutamate receptor;Intermediates & Fine Chemicals;API;TOPAMAX;Pharmaceutical raw materials |
| Mol File: | 97240-79-4.mol |
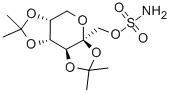 |
|
| Topiramate Chemical Properties |
| Melting point | 125°C |
| alpha | D23 -34.0° (c = 0.4 in methanol) |
| Fp | 9℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: ~44 mg/mL |
| form | solid |
| color | white |
| Merck | 14,9547 |
| Safety Information |
| Hazard Codes | Xi,T,F |
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36-45-36/37-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | LS7083000 |
| Hazardous Substances Data | 97240-79-4(Hazardous Substances Data) |
| Topiramate Usage And Synthesis |
| Broad-spectrum anti-epileptic drugs | Topiramate (the TPM) is a naturally existing monosaccharide D-fructose sulfide, and together with felbamate, lamotrigine and vigabatrin are current several broad-spectrum anti-epileptic drugs with relatively wide clinical application and can be used to control different types of epilepsy with excellent efficacy and pharmacokinetics. But in cases of being applied to children or fast increase of the amount can cause cognitive impairment and neurotoxicity, and easy to trigger kidney stones. In 1980, scientists had first successfully synthesized tipiramate in the laboratory. It had been applied to patients with epilepsy for the first time in 1986. In 1995, it was approved for entering into the market of UK for the first time. Its basic structure is fructopyranose sulfamate. Unlike other kinds of anti-epileptic drug, TPM has various kinds of anti-epileptic mechanisms including blocking the voltage-dependent sodium channels, enhancing the activity of GABA in the location of the γ-aminobutyric acid A (GABAA) receptor as well as blocking the activity of the AMPA glutamate receptor. In addition, there is still mild effect of carbonic anhydrase inhibitors. Europe and the United States had conducted double-blind, placebo-controlled studies and demonstrated that the adjunctive therapy with topiramate has excellent efficacy, safety and is well tolerated in the treatment of various types of refractory epilepsy. Now it has begun with topiramate monotherapy and has also achieved good results with 62% of patients with epileptic seizures disappearing completely. This product is generally used for the adjuvant treatment of antiepileptic drugs and is effective in treating simple and complex seizures as well as systemic tonic-clonic seizures and can also be used for the treatment of infantile spasms. Features of this product include excellent long-term efficacy, no significant resistance as well as being able to be used alone for antiepileptic purpose. |
| Pharmacological effects | The major mechanism of Topiramate is through blocking the dispersion of epileptic seizures rather than preventing its occurrence. It has been also found that TPM can exert its efficacy in treating epilepsy through various kinds of mechanisms including: 1, blocking the voltage-dependent sodium channels and thereby reducing the duration of epileptic discharges and the number of action potentials generated during each discharge. 2, antagonizing kainate/AMPA--glutamate receptors. 3, enhancing the GABA activity in the non-benzodiazepine position of the GABA receptor. 4, mildly inhibiting carbonic anhydrase. 5, blocking the T-type calcium channels. 6, block the activity of the excitatory neurotransmitter of the central nervous AMPA glutamate receptor. Recent studies have shown that blocking L-type high-voltage-dependent calcium channels may be one of the most important mechanisms of the action of antiepileptic topiramate. Topiramate (TPM) can be used to prevent the animal epileptic seizures induced by maximum electroshock seizure test (MES) but has no effect on the chemical drug-induced epileptic seizure as well as can’t be used to prevent the occurrence of the epileptic seizures. Electrode physiological studies on the cultured hippocampal neurons have shown that 10μmol/ml topiramate can reduce the incidence of neural ignited spontaneously epileptic seizures and action potentials while 20μmol/ml of TPM can reduce the frequency of action potential firing. The antiepileptic effect of topiramate may also be related to its effect on increasing the GABA-induced influx of the chloride ions. Similar to benzodiazepine, TPM is also capable of increasing the GABA induced penetration of chloride particles through the cultured cell membrane. In addition to affecting the flow of chloride ions, TPM also increase the frequency of CABA’s activation of GABAA receptor. However, the activation is not through the interaction of the GABA binding site or benzodiazepine binding site. Topiramate has also been found to have mild inhibitory effect on the two carbonic anhydrase isozymes: carbonic anhydrase II and carbonic anhydrase IV. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Pharmacokinetics | Topiramate is a white crystalline powder with bitter taste and is easily soluble in alkaline solution. Its saturated solution has a pH of 6.3. It can be rapidly absorbed after oral administration with achieving the average Cmax = 1.5 μg/ml within 2 to 3 hours (Tmax). Food had no significant effect on the bioavailability of topiramate with the oral absorption averaged on about 81%. There is 13% to 17% of topiramate binding to the plasma proteins with the average volume of distribution being 0.55~0.80L/kg. Upon single oral administration of a dosage of 100~400mg, it exhibits a linear drug-metabolism property. Patients with normal renal function can have it reach steady-state plasma concentrations in 4 to 8 days. Oral administration of 50 mg and 100 mg 2 times per day has the average T1/2 of approximately 21 hours. Oral administration of 100~400mg with 2 times per day together with taking phenytoin or carbamazepine can increase the plasma concentrations of the latter two drugs in positive dose-dependent relationship. Therefore, such patients should be subject to close observation on the adverse reactions and monitoring of the drug plasma concentrations when necessary. Instead, carbamazepine and phenytoin sodium can reduce the plasma concentration of topiramate. Therefore we need to adjust the dose based on efficacy and adverse reactions. Valproate doesn’t significantly affect the plasma concentration of topiramate. Only about 20% of the topiramate can be subject to metabolism in its prototype. When used in combination with antiepileptic drug for treatment, about 50% of the topiramate is converted by metabolic enzymes. The six kinds of topiramate metabolites produced by the body all have no obvious anticonvulsant activity. 80% of the prototype topiramate in the body as well as its metabolites are subject to renal clearance. Oral administration of 50~100mg for 2 times per day gives the average renal clearance rate being about 18ml/min. Patients of renal impairment or hepatic injury has a decreased plasma clearance and renal clearance rate and the time for reaching steady state plasma concentration may take 10 to 15 days. Usually elderly patients have their plasma clearance rate be unchanged. |
| Dosage | It acts as adjuvant drugs for the treatment of partial epileptic seizure with or without secondary systemic seizure. It is preferably to start administrating it from a low dose and gradually increase to the effective dose. Do not crush the tablets. For adults, it is recommended to take 50 mg per night during the first wee; take 50 mg at both day and night during the second week; take 50 mg in the morning and 100 mg in the evening; take 100 mg at both day and night during the fourth week; take 100 mg at day and take 150 mg at night in the fifth week; take 150 mg at both day and night during the sixth week; take 150 mg at day and 200 mg at night during the seventh week; take 200 mg at both day and night at the eighth week. Maintenance dose: 400mg/d. For children of 2 to 16 years old, the recommended dosage is 5~9mg/kg daily and divided into 2 times. The dose should be adjusted to 25mg (day 1~3 mg/kg) at night during the first week and then add 1~3mg/kg every day at the interval of 1 to 2 weeks with administration in 2 times until reaching the optimal clinical efficacy. |
| Clinical evaluation | 33 cases of patients of epilepsy apply monotherapy or combination treatment with other antiepileptic drugs with the initial dose of 25mg, qd; the other 40 patients were subject to carbamazepine treatment as control. The effective rate of the topiramate treatment group was 93.9% which is significantly higher than that of the carbamazepine treatment group 77.5% (P <0.05); topiramate monotherapy has a better efficacy than combination therapy. In preclinical tests of the AEDs development of the National Institutes of Health (NIH), researchers had studied the efficacy of topiramate as adjuvant therapy in the treatment of adult epilepsy patients. 41% of patients treated with topiramate had their seizure times decreased> 50% while the value in the placebo group was only 10%; 19% of patients treated with topiramate get the seizure times decreased> 75% (the value is only 3% in the placebo group). |
| Adverse reactions and precautions | 1, since topiramate is often used in combination with other anti-epileptic drugs, it is therefore difficult to distinguish which drug or several drugs are related to the adverse reactions. The most frequently reported adverse reactions drug are symptoms associated with the central nervous system including ataxia, impaired attention, confusion, dizziness, fatigue, paresthesia, somnolence and abnormal thinking. This is potential risk factors for patient in driving or operating machinery. Common adverse reactions include anxiety, forgetting, loss of appetite, aphasia, depression, diplopia, mood swings, nausea, nystagmus, verbal expression disorder, taste perversion, abnormal vision and weight loss. Occasionally there are reports of renal stone disease. Drinking lots of water during the treatment can reduce the risk factors. Patients known allergic to the chemicals should be disabled. 2, similar to other anti-epileptic drugs, we should discontinue it gradually so that the possibility of increased seizure frequency can be reduced to minimum. In clinical trials, the reduced amount weekly is 100mg/day. 3, same as with other antiepileptic drugs, animal experiments confirmed that topiramate has teratogenic effects. 4, upon acute overdose, if ingested just now, you should immediately adopt gastric tube induced gastric emptying or induced vomiting for gastric emptying for rescue. Activated carbon does not adsorb topiramate, therefore it is not recommended to apply it upon overdose. Apply hemodialysis if necessary. 5, when using in combination phenytoin, we should monitor with the phenytoin plasma concentrations. Phenytoin and carbamazepine can reduce the plasma concentration of this product. For patients taking digoxin, when adding or discontinuing this product, pay attention to monitoring the digoxin plasma concentration. When topiramate is used in combination with oral contraceptives, the efficacy of the contraceptive may be reduced. You may need to adjust the dose of this product when added with hydrochlorothiazide. It is not recommended to administer together with alcohol or other central nervous system depressants. For patients being subject to metformin treatment, if increasing or stopping the treatment of this product, it should be closely monitored of the diabetic condition. Upon being used combination with pioglitazone, we should pay attention to the control of the diabetes disease. Being used in combination with other drugs which can lead to kidney stones can increase the risk of kidney stones. Overdose may cause convulsions, drowsiness, speech disorder, blurred vision, diplopia, mental impairment, lethargy, ataxia, stupor, hypotension, abdominal pain, dizziness and depression. Topiramate could result in severe metabolic acidosis. |
| Chemical Properties | It is fine white powder crystals. |
| Chemical Properties | White-to-Off-White Crystalline Powder |
| Uses | anticonvulsant, antimigraine, GABA-A agonist, AMP/kinate glutamate receptor antagonist, carbonic anhydrase inhibitor |
| Uses | Used for the treatment and control of partial seizures and severe tonic-clonic (grand mal) seizures and also for the prevention of migraine headaches. In children it is also used for treatment of Lennox-Gastaut syndrome. Qsymia? is indicated for the treat |
| Uses | Used as an anticonvulsant |
| Definition | ChEBI: A hexose derivative that is 2,3:4,5-di-O-isopropylidene-beta-D-fructopyranose in which the hydroxy group has been converted to the corresponding sulfamate ester. It blocks voltage-dependent sodium channe s and is used as an antiepileptic and for the prevention of migraine. |
| Biological Activity | Anticonvulsant. Antagonizes GluR5 kainate receptors (IC 50 = 0.46 μ M), acts as a positive allosteric modulator of GABA A receptor-mediated currents, inhibits Na v channels (IC 50 = 48.9 μ M) and inhibits L-type Ca 2+ channels. Also inhibits carbonic anhydrase (CA) (K i values are 0.1 and 0.2 μ M at rat CA II and CA IV respectively), which lowers intracellular neuronal pH. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Enaltec Labs Pvt Ltd
- 97240-79-4 Topiramate 99%
- Inquiry
- 2024-03-21
-
VIP1年
- Beloorbayir Biotech Ltd
- Topiramate 97240-79-4 98%
- Inquiry
- 2024-03-19
-
VIP1年
- Unimark Remedies Ltd
- Topiramate 99%
- Inquiry
- 2024-03-16
-
VIP1年
- SLS Pharmaceuticals Private Limited
- 97240-79-4 Topiramate 98%
- Inquiry
- 2024-03-13
-
VIP1年
- Bal Pharma Limited
- 97240-79-4 98%
- Inquiry
- 2024-02-20
-
VIP1年
- Atul Bioscience Ltd
- 97240-79-4 Topiramate 99%
- Inquiry
- 2024-02-05
-
VIP1年
- Ralington Pharma
- Topiramate 97240-79-4 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Raising Sun Pharma
- Topiramate 98%
- Inquiry
- 2024-02-03
-
VIP1年
- Amara Labs Pvt Ltd
- 97240-79-4 98%
- Inquiry
- 2024-01-29
-
VIP1年
- Global Calcium Pvt Ltd
- 97240-79-4 Topiramate 99%
- Inquiry
- 2024-01-27
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
