

Tazarotene
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameTazarotene
- CAS No.118292-40-3
- EINECS No.601-516-4
- MFC21H21NO2S
- MW351.46
- Appearancepowderwhite to very faintly yellow
- storage temp. 2-8°C
- Melting point 95-98°C
- Boiling point 499.8±45.0 °C(Predicted)
- density 1.22±0.1 g/cm3(Predicted)
AD68
| Tazarotene Basic information |
| Product Name: | Tazarotene |
| Synonyms: | 3-pyridinecarboxylicacid,6-((3,4-dihydro-4,4-dimethyl-2h-1-benzothiopyran-6-y;agn190168;ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]pyridine-3-carboxylate;TAZAROTENE;6-[2-(3,4-Dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl]- 3-pyridinecarboxylic Acid Ethyl Ester;Tazorac;Zorac;6-[(3,4-Dihydro-4,4-dimethyl-2H-1-benzothiopyran)-6-yl]nicotinic acid ethyl ester |
| CAS: | 118292-40-3 |
| MF: | C21H21NO2S |
| MW: | 351.46 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Retinoids;Sulfur & Selenium Compounds;Heterocycles;API;Tazorac, Avage, Zora;Inhibitors |
| Mol File: | 118292-40-3.mol |
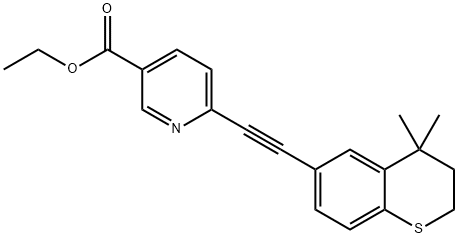 |
|
| Tazarotene Chemical Properties |
| Melting point | 95-98°C |
| storage temp. | Store at +4°C |
| solubility | DMSO: >15mg/mL |
| color | white to very faintly yellow |
| Merck | 14,9081 |
| CAS DataBase Reference | 118292-40-3(CAS DataBase Reference) |
| Safety Information |
| RTECS | US5675100 |
| Tazarotene Usage And Synthesis |
| Description | Tazarotene belongs to a third generation prescription topical retinoid. It is marketed in different forms including cream (brand name: Avage), foam (brand name: Fabuir) and gel (brand name: Tazorac). It is mainly used for the treatment of psoriasis, acne, and sun damaged skin. The detailed mechanisms of action of Tazarotene are still unclear. It is converted to its active form, the cognate carboxylic acid of tazarotene (AGN 190299) in vivo through rapid de-esterification in animals and man. AGN 190299 is capable of binding to all the three members of the retinoic acid receptor (RAR) family: RARα, RARβ, and RARγ with relative selectivity for RARβ, and RARγ. This process may further modify gene expression. However, it is unclear whether this is related to its mode of action. Common side effects associated with it include worsening of acne, desquamation, burning/stinging, erythema and pruritus increased sensitivity to sunlight, dry skin, itchiness, redness, skin pain rash and skin discoloration. In most cases, these side effects are mild and can remarkable decrease after the first 2–4 weeks. Tazarotene should not be used in the following cases: patients are allergic to it; patients are pregnant; patients have a sunburn; patients are taking photo-sensitive drugs such as thiazides and tetracycline. |
| Dermatology drugs | Tazarotene belongs to the third generation retinoid dermatology drug and is also the retinoid A-like prototype for skin tropical administration and has regulatory effect on epidermal cell differentiation and proliferation. It is mainly used for the treatment of plaque psoriasis vulgaris. Tazarotene cream can also be used for the treatment of facial acne. The clinical administrated product contains an active ingredient of this product being 15mg/30g with its gel being subject to tropical administration. At half an hour before sleep every night, apply appropriate amount of this product into the wounded part. Before treatment, first clean the affected area and apply the drug evenly to the skin lesion part after the dry of the skin for forming a thin film; after applying the drug, the patients should gently rub in order to promote the absorption of drugs. Pea-sized gel can cover a palm-size area of skin lesions. The application area should not exceed 20% of body surface area. Wash hands with soap after rubbing the drug. Psoriasis is a common benign, acute or chronic inflammatory skin disease. It occurred on the basis of genetic predisposition. Trauma or irritation of normal skin can induce the occurrence of psoriasis lesions (Koebner phenomenon): There are several different manifestations of psoriasis-the most common is plaque type. Rash type (guttate) psoriasis includes a lot of 3~10mm skin lesions. It may occasionally occur after mental stress or streptococcal pharyngitis. Seriously, there may be occasionally life-threatening forms such as generalized pustular and erythrodermic psoriasis. Sudden onset of plaque type or generalized erythrodermic psoriasis may be associated with HIV infection. Tazarotene gel belongs to the topical formulations of formic acid and can be used for the treatment of psoriasis. Early research has shown that: twice daily for topical administration of eight weeks with about 50% patients with mild to moderate psoriasis having at least 75% of their lesions be alleviated. But the completely eliminated plaque number accounts for less than 10%. Tazarotene gel accounts for concentrations of 0.05% and 0.1%. There is no difference on the efficacy of topical administration of 0.1% gel once per day or twice per day. However, tropical administration of the 0.5% gel once per day gives a poor efficacy. Tazarotene, as a synthetic ethylene retinal, after topical application, it can be quickly converted to tazarotene acid and plays the pharmacological effects. Tazarotene acid, after binding to the skin retinoid acid receptor-γ (RAR-γ), it can achieve the purpose of alleviating the clinical symptoms through restoring to normal skin differentiation and reducing the inflammation of the skin. |
| Adverse reactions and precautions | Adverse reactions of tazarotene mainly include skin reactions, manifested as itching, burning, stinging, redness, irritation, skin irritation, scaling, dermatitis, chapped, edema, bleaching, drying and bleeding. Precautions: 1. For women of childbearing age, within the first 2 weeks before the start of tazarotene gel treatment, we need to perform serum or urine pregnancy test to confirm that the pregnancy test is negative. Start the treatment at the secondary day or third day in the next normal menstrual cycle. Before treatment, during treatment and for some time after cessation of treatment, the patients must use effective contraceptive methods. 2. Avoid contact between drug and eyes, mouth and mucous membrane; try to avoid the contact between skin and the drug. In case of contact with eyes, rinse thoroughly with water. 3. In cases of skin irritation such as itching, try not to scratch and can apply with a small amount of emollient; in severe case, you should stop using this drug or administer at the next day. 4. This product is not recommended for treating acute eczema. 5. During the treatment, avoid excessive exposure to the sun. You should also avoid simultaneously administration of taking photosensitive drug. 6. After application of the drug, avoid using clothing and bandaging for affected area. You should avoid using pharmaceuticals and cosmetics which can dry the skin. Safety information regarding to the applied area should not exceed 20% of the body surface area has not been established. 7. It should be contraindicated in pregnant and lactating women. For this drug, patients allergic to other aldehydes retinol or vitamin A derivative should be tabooed. 8. Safety and efficacy regarding to the children under age 12 for using tazarotene has not been established. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Chemical Properties | It is as white-like or pale yellow solid. |
| References | https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020600s008lbl.pdf https://en.wikipedia.org/wiki/Tazarotene http://www.rxlist.com/tazorac-drug/side-effects-interactions.htm |
| Chemical Properties | White Solid |
| Uses | An acetylenic retinoid prodrug converted to the active metabolite, Tazarotenic acid, with selective affinity for retinoic acid receptors RAR?and RAR. Antiacne; antipsoriatic. Used in treatment of photodamaged skin |
| Uses | An acetylenic retinoid prodrug converted to the active metabolite, Tazarotenic acid, with selective affinity for retinoic acid receptors RARβ and RARγ. Antiacne; antipsoriatic. Used in treatment of photodamaged skin. |
| Uses | Tazarotene is a prescription topical retinoid sold as a cream or gel. This medication is approved for treatment of psoriasis, acne, and sun damaged skin (photodamage). It is commonly sold in two concentrations: 0.05% and 0.1%. In addition to tretinoin, wh |
| Uses | anticholelithic |
| Uses | Used to treat psoriasis, acne and sun damaged skin. |
| Definition | ChEBI: The ethyl ester of tazarotenic acid. A prodrug for tazarotenic acid, it is used for the treatment of psoriasis, acne, and sun-damaged skin. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Maithri Drugs Pvt Ltd
- 118292-40-3 Tazarotene 98%
- Inquiry
- 2024-03-28
-
VIP1年
- Enaltec Labs Pvt Ltd
- Tazarotene 99%
- Inquiry
- 2024-03-21
-
VIP1年
- BDR Pharmaceuticals International Pvt Ltd
- Tazarotene 118292-40-3 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Laxai Life Sciences
- Tazarotene 98%
- Inquiry
- 2024-01-18
-
VIP1年
- PANCHSHEEL ORGANICS LTD
- TAZAROTENE 99%
- Inquiry
- 2023-12-29
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
