

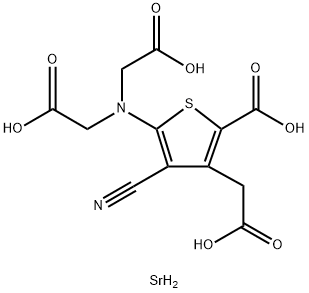
Strontium ranelate
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameStrontium ranelate
- CAS No. 135459-87-9
- EINECS No.690-882-9
- MFC12H12N2O8SSr
- MW431.91
- Appearancepowderwhite to beige
- Melting point >310°C (dec.)
- storage temp. Inert atmosphere,Store in freezer, under -20°C
AD68
| Strontium ranelate Basic information |
| Product Name: | Strontium ranelate |
| Synonyms: | Ranelate StrontiuM;StrontiuM ranelate (Protelos);2,2'-((5-Carboxy-4-(carboxyMethyl)-3-cyanothiophen-2-yl)azanediyl)diacetic acid, distrontiuM salt;StrontiuM 2,2'-((5-carboxylato-4-(carboxylatoMethyl)-3-cyanothiophen-2-yl)azanediyl)diacetate;StrontiuM Ranelic;StrontiuM ranelate SynonyMs DistrontiuM renelate;2-[N,N-Di(carboxymethyl)amino]-3-cyano-4-carboxymethylthiophene-5-carboxylic acid strontium salt;Ranelic acid strontium salt |
| CAS: | 135459-87-9 |
| MF: | C12H6N2O8SSr2 |
| MW: | 513.491 |
| EINECS: | 1806241-263-5 |
| Product Categories: | Pharmaceutical intermdiate;API;Other APIs;All Inhibitors;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals |
| Mol File: | 135459-87-9.mol |
 |
|
| Strontium ranelate Chemical Properties |
| Melting point | >310°C (dec.) |
| storage temp. | -20°C Freezer |
| solubility | H2O: soluble1mg/mL, clear (warmed) |
| color | white to beige |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22 |
| Safety Statements | 36/37 |
| RIDADR | 3077 |
| RTECS | XM7581000 |
| Strontium ranelate Usage And Synthesis |
| Drug for the treatment of osteoporosis | Strontium ranelate is a drug for the treatment of osteoporosis with its appearance being white to light yellow powder or crystalline powder. It is odorless and slightly soluble in water but almost insoluble in ethanol and easily soluble in dilute hydrochloric acid. It was first studied and developed by the French Servier Company and had first entered into market in November 2004 in Ireland and entered into market in UK in December of same year. Japan's Fujisawa Pharmaceutical Company has owned the authorization of development, production and marketing right of this product in Japan. It is clinically mainly used for the treatment and prevention of osteoporosis in postmenopausal women with significantly reducing the risk of occurrence of vertebral fractures and hip fractures. Strontium ranelate has dual pharmacological inhibitory effects of both inhibiting bone absorption and promoting bone formation. On the one hand, in the osteoblast-enriched cells, it can increase the synthesis of collagen and non-collagen proteins and promote the osteoblast-mediated bone formation mediated by osteoblasts through enhancing the proliferation of pre-osteoblast. On the other hand, through decreasing the osteoclast differentiation and reabsorbing activity and further reduction of bone absorption, it achieves the rebalance of bone turnover, further boosting the bone formation. Strontium ranelate mainly exerts its pharmacological effects through its strontium atoms. Strontium is an alkaline earth metal element which is cognate with calcium and located under the calcium in the element periodic table. Its absorption, distribution, excretion is similar with calcium. After oral administration of 2g, the absolute bioavailability of strontium is 27%. Large doses of strontium cause abnormalities of bone mineral metabolism with low doses of strontium being able to the enhance the pre-osteoblast replication, increasing the number of osteoblasts to stimulate bone formation while reducing the activity of osteoclasts, reducing osteoclast quantity as well as reducing the rate of bone absorption. The results are consistent with the results found in animal and human in vivo studies. s (OP) is a progressive skeletal disease, characterized by reduced bone mineral density (BMD) and degenerative changes in bone tissue microstructure. It is exhibited as bone fragility and fracture-prone with the latter most commonly happening in the spine, hip and wrist. For women, when after surgical removal of the ovaries or menopause, the body stops producing bone to maintain strong estrogen, thus, primary OP is particularly common in post-menopausal or menopausal women. There are currently two major kinds of drugs used in the treatment of osteoporosis: one kind includes those drugs that inhibits osteoclast activity and therefore inhibiting bone absorption such as bisphosphonates, estrogen and calcitonin; the second category includes those drugs which promote the osteoblast activity, and thereby stimulating bone formation and there are currently only a product, human recombinant parathyroid hormone 1-34, that has entered into market. It however requires injection with high drug prices. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Uses | It is mainly used for the treatment and prevention of osteoporosis in postmenopausal women and significantly reduces the risk of occurrence of vertebral fractures and hip fractures. |
| Chemical Properties | Crystalline Solid |
| Uses | Bone metabolism modulator; inhibits bone resorption while maintaining bone formation. Antiosteoporotic |
| Uses | Strontium ranelate (Protelos) is a strontium(II) salt of ranelic acid for (-)-desmethoxyverapamil binding to calcium channel with IC50 of 0.5 mM. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Maithri Drugs Pvt Ltd
- Strontium ranelate 135459-87-9 98%
- Inquiry
- 2024-03-28
-
VIP1年
- Suntril Pharmaceuticals Pvt Ltd
- 135459-87-9 Strontium ranelate 98%
- Inquiry
- 2024-03-06
-
VIP1年
- Cadchem Laboratories Limited
- Strontium ranelate 135459-87-9 98%
- Inquiry
- 2024-03-06
-
VIP1年
- BDR Pharmaceuticals International Pvt Ltd
- Strontium ranelate 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Ralington Pharma
- 135459-87-9 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Dishman Carbogen Amcis Ltd (Dishman Group)
- 135459-87-9 Strontium ranelate 98%
- Inquiry
- 2024-01-05
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
