

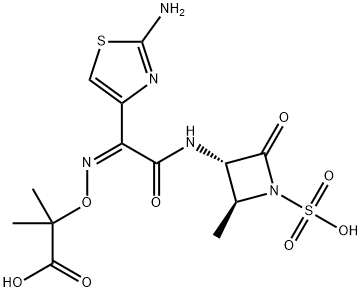
Aztreonam
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameAztreonam
- CAS No.78110-38-0
- EINECS No.278-839-9
- MFC13H17N5O8S2
- MW435.43
- Appearancesolidwhite to beige
- density 1.83
- Melting point 227°C
- storage temp. room temp
- Water Solubility Soluble in DMF/water (1:1) at 50 mg/ml
AD68
| Product Name: | Aztreonam |
| Synonyms: | Aztreonam L-Arginine;(E)-2-(((1-(2-aminothiazol-4-yl)-2-((2-methyl-4-oxo-1-sulfoazetidin-3-yl)amino)-2-oxoethylidene)amino)oxy)-2-methylpropanoic acid;Aztreonam/arginine;Aztreonam(β-form);Aztreonam94-103% (HPLC);Aztreonam and Sodium Carbonate;(2s-(2-alpha,3-beta(z)))-dinyl)amino)-2-oxoethylidene)amino)oxy)-2-methyl;antibioticsquibb26,776 |
| CAS: | 78110-38-0 |
| MF: | C13H17N5O8S2 |
| MW: | 435.43 |
| EINECS: | 278-839-9 |
| Product Categories: | API;TISERTON;Active Pharmaceutical Ingredients;Antibacterial;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;Heterocycles;Sulfur & Selenium Compounds;Aztreonam ada@tuskwei.com whatsapp;8618031153937 |
| Mol File: | 78110-38-0.mol |
 |
|
| Aztreonam Chemical Properties |
| Melting point | 227°C |
| density | 1.83 |
| refractive index | 1.6460 (estimate) |
| storage temp. | Store at 0-5°C |
| form | solid |
| Water Solubility | Soluble in DMF/water (1:1) at 50 mg/ml |
| Merck | 14,925 |
| InChIKey | WZPBZJONDBGPKJ-VEHQQRBSSA-N |
| CAS DataBase Reference | 78110-38-0(CAS DataBase Reference) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 2 |
| RTECS | UA2451400 |
| HS Code | 29419000 |
| Aztreonam Usage And Synthesis |
| Outline | Aztreonam is a novel kind ofβ-lactam antibiotics belonging to monobactams. In 1978 it was first discovered from the culture brot of New Jersey soil purple bacterium Chromobacterium violaceum, it has been obtained with synthesis method. Mechanism Aztreonam belongs to bacteria fungicides. It can quickly go through the outer membrane of Gram-negative aerobic bacteria wall, while it has a high affinity with the penicillin-binding protein 3 (PBP-3) . By acting on the PBP-3 and inhibiting synthesis of bacterial cell wall , it leads to cell lysis and death. Aztreonam has a narrow antimicrobial spectrum, it only has antibacterial effect on aerobic gram-negative bacilli , such as E. coli, Klebsiella, Serratia spp, Proteus mirabilis, indole-positive Proteus, Citrobacter, influenza addicted blood coli, Pseudomonas aeruginosa and other Pseudomonas, some Enterobacter, Neisseria gonorrhoeae. The product is highly stable to β-lactamase produced by many bacteria .it is inactive in Gram-positive bacteria and anaerobic bacteria . Aztreonam compared with ceftazidime, gentamicin, it effect on aerogenes, and Enterobacter cloacae is higher than ceftazidime, but lower than gentamicin; the effect on Pseudomonas aeruginosa is lower than ceftazidime , and similar to gentamicin . |
| indications | It is mainly used for the treatment of infections caused by susceptible strains which include the respiratory system, urinary, reproductive system infections (including acute gonorrhea), intra-abdominal infections, skin and soft tissue infections, before surgery preventing infections, other serious infections, such as sepsis. |
| Precautions | 1.contraindication: patients allergic to the products or having a history of allergy on lidocaine and other local anesthetics or having anaphylactic shock on other β-lactam drugs are banned. 2. caution following: It does not exist cross-allergic reactions between aztreonam and penicillins, but people having allergy body and allergic to penicillins, cephalosporins should take caution. renal dysfunction patients should take caution. aztreonam does not have toxicity for liver , but the patients having impaired liver function should observe the dynamic changes. It has been reported that in patients with alcoholic cirrhosis , the total clearance of the products can be reduced by 20-25%. pregnant and lactating women, infants and young children should use with caution. 3. The impact of drugs on clinical outcomes: direct anti-human globulin test (Coombs test) can be positive. there may be a temporary increase of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and serum creatinine values after medication among a few patients, part thromboplastin time (PTT) and prothrombin time (PT) may be extended. adverse reactions are skin symptoms such as skin rashes, purpura, pruritus (approximately 2%); gastrointestinal symptoms such as diarrhea, nausea, vomiting, taste changes, jaundice, and drug-induced hepatitis (approximately 2%); local irritation such as thrombophlebitis. Injection site swelling (approximately 2.4%); there are other neurological symptoms, vaginitis, oral lesions, fatigue, dizziness, and bleeding. 4. Long-term medication should pay attention to monitoring of liver, kidney and hematopoietic system. The above information is edited by the chemicalbook of Tian Ye. |
| Chemical properties | white or colorless powder crystals, melting at 227 ℃ (decomposition). It is dissolved in dimethyl formamide, slightly soluble in methanol, very slightly soluble in ethanol, insoluble in toluene, chloroform or ethyl acetate. Aztreonam disodium: C13 H15N5Na2O8 . Acute toxicity LD50 (mg/kg): 3300 intravenous injection in mice, 6600 in rats intraperitoneally. |
| Uses | It is Mainly used for the infections caused by sensitive gram-negative bacteria , including pneumonia, pleurisy, abdominal infections, biliary tract infections, bone and joint infections, skin and soft tissue inflammation, especially for urinary tract infections, but also for sepsis. Because this product has good resistance to enzyme performance, therefore, when a microorganism is not sensitive to penicillins, cephalosporins, aminoglycosides and other drugs ,the product should often be effective. |
| production method | Threonine for raw materials,chloride resulting from the chlorination forms an amide through ammonolysis, protect α-amino with benzyl chloroformate,esterifyβ-hydroxy with methanesulfonyl chloride , the amide group is sulfonated with sulfuric acid tetrabutylammonium , then it is cyclized to generate azetidine derivative in the potassium bicarbonate, then after hydrogen and Deprotection, produce 3-amino-2-methyl-4-oxo-1-sulfo-N oxetane. Afetr dehydration amidation of side chains Azetidine derivative and thiazole derivatives obtained in the above, hydrolyse to deprotect and to obtain aztreonam. |
| Category | Toxic substances |
| Toxicity grading | Middle toxic |
| Acute toxicity | Intraperitoneal-rat LD50: 2549 mg/kg; intraperitoneal-Mouse LD50: 2897 mg/kg. |
| Flammability and hazard characteristics | Combustible; combustion produces toxic fumes of nitrogen oxides and sulfur oxides. |
| Storage Characteristics | Ventilated, low-temperature ,dry storeroom. |
| Extinguishing agent | Dry powder, foam, sand, carbon dioxide, water spray. |
| Chemical Properties | White Crystalline Powder |
| Uses | The first totally synthetic monocyclic β-lactam antibiotic. |
| Uses | antiserotonin |
| Uses | The first totally synthetic monocyclic ?lactam antibiotic |
| Uses | Aztreonam is a synthetic β-lactam antibiotic of the monobactam class. It is effective against Gram-negative bacteria but inactive against Gram-positive bacteria. Its mechanism of action involves the inhibition of mucopeptide synthesis in the bacterial cell wall, thereby blocking peptidoglycan crosslinking. Aztreonam is resistant to hydrolysis by some β-lactamases, but is inactivated by extended-spectrum β-lactamases. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- PROTECH TELELINKS
- 78110-38-0 98%
- Inquiry
- 2024-02-09
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
