

Vancomycin hydrochloride
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameVancomycin hydrochloride
- CAS No.1404-93-9
- EINECS No.604-193-8
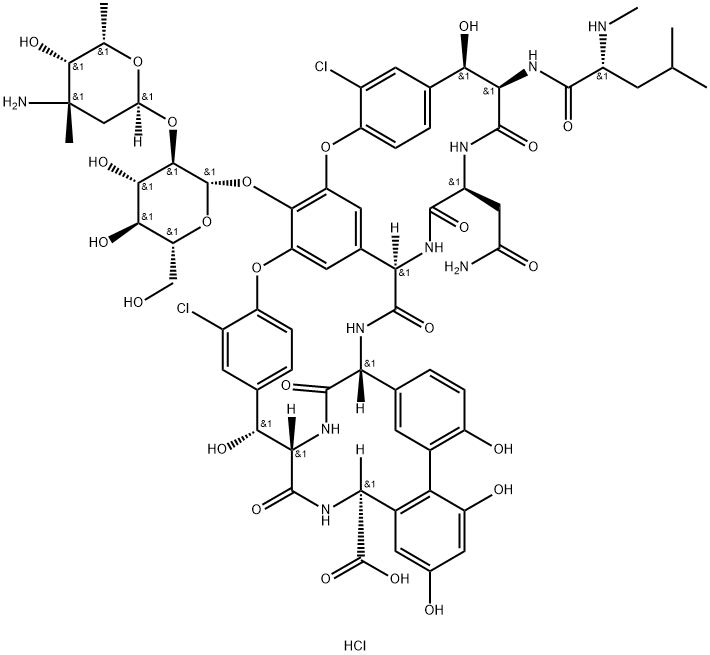
- MFC66H76Cl3N9O24
- MW1485.72
- AppearancePowdercolorless to faint yellow or tan
- storage temp. 2-8°C
- Melting point >190°C (dec.)
- Water Solubility Soluble in water. Slightly soluble in methanol, ethanol and dimethylsulfoxide.
AD68
| Vancomycin hydrochloride Basic information |
| Product Name: | Vancomycin hydrochloride |
| Synonyms: | vancocinehydrochloride;LYPHOCIN;VANCOMYCIN, HYDROCHLORIDE, STREPTOMYCES ORIENTALIS;VANCOR;VANCOCIN;VANCOCIN HYDROCHLORIDE;VANCOMYCIN HCL;VANCOMYCIN HCL, STREPTOMYCES ORIENTALIS |
| CAS: | 1404-93-9 |
| MF: | C66H76Cl3N9O24 |
| MW: | 1485.71 |
| EINECS: | 215-772-6 |
| Product Categories: | Antibacterial;Intermediates & Fine Chemicals;Pharmaceuticals;Antibiotics and Antimycotics;GlycopeptidesAntibiotics;Antibiotics A to;Antibiotics T-ZAntibiotics;AntibioticsAntibiotics;Chemical Structure Class;Interferes with Cell Wall SynthesisSpectrum of Activity;L - Z;Mechanism of Action;Plant Tissue Culture;L - ZPharmacopoeia (USP);Core Bioreagents;Pharmacopoeia A-ZPharmacopoeia (USP);Pharmacopoeial AntibioticsMore...Close...;Research Essentials;Antibiotics;API's;Peptide Synthesis/Antibiotics;Chiral Reagents;Metabolites & Impurities;API;VIRA-A;antibiotic |
| Mol File: | 1404-93-9.mol |
 |
|
| Vancomycin hydrochloride Chemical Properties |
| Melting point | >190°C (dec.) |
| Fp | 87℃ |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, yellow |
| form | Powder |
| color | colorless to faint yellow or tan |
| Water Solubility | Soluble in water. Slightly soluble in methanol, ethanol and dimethylsulfoxide. |
| Sensitive | Hygroscopic |
| Merck | 13,9995 |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 43-42 |
| Safety Statements | 36/37-24/25-22-36 |
| WGK Germany | 2 |
| RTECS | YW4380000 |
| F | 8-10-21 |
| Toxicity | LD50 in mice (mg/kg): 489 i.v.; 1734 i.p.; 5000 s.c.; 5000 orally (Anderson) |
| Vancomycin hydrochloride Usage And Synthesis |
| Glycopeptide antibiotics | Vancomycin hydrochloride is a glycopeptide antibiotic and is the hydrochloride salt of vancomycin. It is white or white-like crystalline powder at room temperature. Its mechanism of action is that it can bind with high affinity to the poly-terminus alanyl-alanine of the precursor peptide located on the cell wall the sensitive bacterial cells, blocking the biosynthesis of the peptide glycan polymer constituting the bacterial cell wall, and thus resulting in the defects of cell wall and further killing bacteria. In addition, it is also possible to change the permeability of the bacterial cell membrane, and selectively inhibit the synthesis of RNA. The characteristic of vancomycin hydrochloride is its strong bactericidal effect against Gram-positive bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and streptococcus pneumoniae. It also has certain anti-bacteria effects on Streptococci anaerobius, Clostridium difficile, Bacillus anthracis, Actinomycetes, Corynebacterium diphtheria, Neisseria gonorrhoeae, Streptococcus viridans, Streptococcus bovis, and Streptococcus faecalis. However, for most Gram-negative bacteria, Mycobacterium, Rickettsia genus, Chlamydia or fungi, it is invalid. It is clinically applicable to the treatment of infection caused by methicillin-resistant Staphylococcus aureus and other bacteria: sepsis, endocarditis, osteomyelitis, arthritis, burns injury, surgical trauma and other superficial secondary infection, pneumonia, lung abscess, empyema, peritonitis, meningitis, pseudomembranous colitis, and skin and soft tissue infections. It is the primary choice for patients who are allergic to penicillin and suffer from the enterococcal endocarditis and Corynebacterium (class diphtheria sp) endocarditis. |
| Vancomycin | Vancomycin belongs to ploy-peptide antibiotics. It is a kind of glycopeptide antibiotics originate from the Streptomyces Orientalis or Amycolatopsis Orientalis. At the late 1950s, the drug had emerged due to its excellent efficacy in treating penicillin-resistant Staphylococcus and because a strong “trump card” antibiotics of anti-G + bacteria including Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae and so on. Subsequently, owing to the discovery of its relative high toxicity, together with the marketing of less-toxic anti-staphylococcal semi-synthetic penicillin and cephalosporin in 1960s and then not very sharp issue of drug resistance, its application was limited. In recent years, because of the increases in cases of infection of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis as well as the demonstration of Clostridium difficile (CD) as the major reason causing antibiotic-associated pseudomembranous colitis, the application cases of vancomycin have been increasing which re-establish the special status of vancomycin in clinical practice. Since 1990s, vancomycin has been always regarded by international experts of antibiotics as "the last line of defense against human intractable drug-resistant strains". From the results of some literature and the distribution of current clinical drug-resistant strains, vancomycin is the primary choice of drug for treating diseases caused by the infection of the MRSA and MRSE. The efficacy of vancomycin in treating CD-associated diarrhea or pseudomembranous colitis is also quite positive, especially for severely sick patients; in addition, for the severe infection caused by the rare penicillin-resistant pneumococcal infections G and penicillin-resistant Corynebacterium JK strain, vancomycin is also a top-grade drug for treating it. Owing to the good efficacy of vancomycin in treating various kinds of diseases caused by drug-resistant bacteria, its marketing sales in nearly 10 years has kept increasing year by year. A few years ago, the increasing rate of international market of vancomycin was maintained at 3-4%, and has risen to 5-6% in the recent two years. According to the estimation of antibiotic expert, the global output in the middle of 1990 s of averaged 20-25 tons per year, and this value had risen to 30 tons in 1999. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Pharmacokinetics | It is poor absorbed through oral administration. Intravenous administration can make it be widely distributed in most body tissues and fluids. Intravenous infusion of 500 mg and 1g yields a peak plasma concentration being 10-30mg/L and 25-50mg/L, respectively. The volume of distribution of the drug is 0.43-1.25L/kg. Its effective antimicrobial concentrations can be achieved in the serum, pericardium, pleura, peritoneum, ascites, and synovial fluid but not in the bile. Vancomycin hydrochloride can penetrate through the placenta but not be able to quickly penetrate through the normal blood-brain barrier and can be introduced into cerebrospinal fluid to reach effective antimicrobial concentration only upon meninges inflammation. The protein binding rate of this drug is about 55%. Its elimination half-life in adults is 6 hours (4-11 hours) in average and can extend to 7.5 day in patients with severe renal insufficiency; for children, it is about 2-3 hours. The drug is metabolized through the liver with about 80%-90% being excreted through renal within 24 hours in its prototype and a small amount being excreted through milk and bile. Hemodialysis or peritoneal dialysis is not able to effectively remove the drug; however, it has been reported that blood perfusion or blood filtration can improve the clearance rate. |
| Dosing instructions | 1. this drug has a strong irritation effect on the tissue is not suitable for intramuscular or intravenous injection; intravenous infusion should try to avoid liquid leakage. 2. In order to reduce the incidence of adverse reactions (such as "red neck syndrome", thrombophlebitis), the intravenous infusion rate should not be too fast with each infusion time beinng at least 1 hour or more. 3. for the treatment of staphylococcal endocarditis, the treatment period should be not less than 28 days. 4. vancomycin hydrochloride is incompatible for being used in combination with chloramphenicol, heparin, aminophylline, sodium bicarbonate, steroid hormones, methicillin, medicines containing heavy metals, and other alkaline solution. 5. overdose treatment: excessive administration of vancomycin hydrochloride can cause oliguria and renal failure. Treatment process comprising: (1) symptomatic and supportive treatment. (2) Conventional hemodialysis and peritoneal dialysis is ineffective in clearing the drugs; but blood perfusion or blood filtration can increase the drug clearance rate. 6. preparation of the solution: (1) Preparation of oral solution: every bottle containing 500 mg of vancomycin should be diluted with distilled water to prepare 500mg/6ml solution for oral administration, the oral solution can be stored for 14 days at 4 °C refrigerator. (2) Preparation of intravenous fluid infusion: ① intermittent infusion, the solution is prepared with 500mg drugs and 10 ml water, and then add it to a 5% glucose injection or 0.9% sodium chloride injection for dilution to less than 5mg/ml before the infusion. The infusion time of a dose of 500mg should be at least 60 minutes and should be at least 100 minutes for a dose of 1000 mg. For patients who is necessary to subject to restricted liquid amount, the highest concentrations can be up to 10mg/ml. ② Upon continuous intravenous infusion, 1~2g dose needs to be added to a sufficient amount of 5% glucose injection or 0.9% sodium chloride injection. 7. the maintenance dose for patients of renal dysfunction can be calculated as follows: the maintenance dose (md/d) = 150 + (15 × creatinine clearance rate of the patient ml/min). 8. plasma concentration should be monitored during treatment; the peak concentration should not exceed 25~40μg/ml, trough concentration should not exceed 5~10μg/ml. Concentration higher than 60μg/ml is within the range of poisoning. If you can’t monitor the blood concentration, adjust the dose according to the creatinine clearance rate. |
| Side effects | The main adverse reactions in clinical application of vancomycin hydrochloride as follows: Ototoxicity: there may be a sense of fullness or tinnitus ear, hearing loss or absence, damage of auditory nerve. In large doses, long time application, the elderly or patients of renal insufficient, it is especially prone to get these symptoms. Renal toxicity: The main damage is on renal tubules. At early stage, there may be proteinuria, urinary tube, followed by hematuria, oliguria, etc; in severe cases, there may be kidney failure. In high dose (plasma concentrations exceed 60~100mg/L), long time application, the elderly or patients of renal insufficient, it is especially prone to get these symptoms. Allergy: upon fast, high-dose of intravenous administration, a small number of patients can get "red neck syndrome." Manifested as chills or fever, fainting, itching, nausea, vomiting, tachycardia, rash or facial flushing; redness or tingling(caused by the release of histamine) in root neck, upper body, back, arm, etc; there may be also occasionally low blood pressure and shock-like symptoms happening. The incidence is higher than norvancomycin and teicoplanin. Local reactions: intramuscular injection or intravenous administration may cause severe pain at the injection site pain with causing thrombophlebitis in severe cases. Gastrointestinal: Oral administration may cause nausea, vomiting, bad smell of mouth and so on. |
| Uses | It has a narrow antimicrobial spectrum which is mainly against Gram-positive bacteria. It is a kind of narrow-spectrum antibiotics only effective in treating gram-positive bacteria, such as Streptococcus pneumoniae, Neisseria gonorrhoeae and enterococci; etc which are all sensitive to this drug. Staphylococcus aureus is particularly sensitive to this product. Its mechanism of action is inhibition of bacterial cell wall synthesis; it mainly binds to the bacterial cell wall and leaving some kinds of amino acids being unable to enter into the glycopeptides of the cell wall. |
| Chemical Properties | off white powder |
| Uses | Amphoteric glycopeptide antibiotic produced by Streptomyces orientalis discovered in soil. Inhibits bacterial cell wall synthesis by binding to peptidoglycan. Antibacterial. |
| Uses | antiviral |
| Uses | Vancomycin hydrochloride is the salt of a glycopeptide antibiotic isolated from Amycolatopsis orientalis in 1956. Vancomycin exhibits potent activity against Gram positive bacteria and is highly effective against MRSA in vitro and in vivo. Vancomycin interferes with cell wall synthesis by binding to D-alanine-D-alanine residues. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Concord Biotech Limited
- Vancomycin hydrochloride 98%
- Inquiry
- 2024-03-30
-
VIP1年
- Ralington Pharma
- 1404-93-9 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Medi Pharma Drug House
- Vancomycin HCL 99%
- Inquiry
- 2023-12-28
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
