


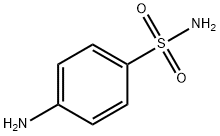
Sulfanilamide
| Price | USD1.00 |
| Packge | 1KG |
- Min. Order:1G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameSulfanilamide
- CAS No.63-74-1
- EINECS No.200-563-4
- MFC6H8N2O2S
- MW172.2
- InChIKeyFDDDEECHVMSUSB-UHFFFAOYSA-N
- Appearancepowder white to faintly beige
- Melting point 164-166 °C(lit.)
- Water Solubility 7.5 g/L at 25 ºC
- density 1.08
- storage temp. 2-8°C
- Boiling point 400.5±47.0 °C(Predicted)
AD68
| Sulfanilamide Basic information |
| Product Name: | Sulfanilamide |
| Synonyms: | p-amino-benzenesulfonamid;p-Aminobenzensulfonamide;p-Aminophenylsulfonamide;Prontalbin;Prontosil album;Prontosil i;Prontosil white;prontosilalbum |
| CAS: | 63-74-1 |
| MF: | C6H8N2O2S |
| MW: | 172.2 |
| EINECS: | 200-563-4 |
| Product Categories: | SULFONAMIDE;Sulfonamides (Antibiotics for Research and Experimental Use);Antibiotics for Research and Experimental Use;Biochemistry;Amines;Intermediates & Fine Chemicals;Metabolites & Impurities;Pharmaceuticals;Sulfur & Selenium Compounds;PRONTYLIN;Amines, Metabolites & Impurities, Pharmaceuticals, Intermediates & Fine Chemicals, Sulfur & Selenium Compounds;pharmaceutical |
| Mol File: | 63-74-1.mol |
 |
|
| Sulfanilamide Chemical Properties |
| Melting point | 164-166 °C(lit.) |
| density | 1.08 |
| refractive index | 1.6490 (estimate) |
| storage temp. | 0-6°C |
| solubility | 5.37g/l |
| form | Liquid |
| color | white to faintly beige |
| PH | 5.8-6.1 (5g/l, H2O, 20℃) |
| Water Solubility | 7.5 g/L at 25 ºC |
| λmax | 257nm(H2O)(lit.) |
| Merck | 14,8925 |
| BRN | 511852 |
| InChIKey | FDDDEECHVMSUSB-UHFFFAOYSA-N |
| CAS DataBase Reference | 63-74-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenesulfonamide, 4-amino-(63-74-1) |
| EPA Substance Registry System | Benzenesulfonamide, 4-amino-(63-74-1) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 40 |
| Safety Statements | 24/25-36-22 |
| WGK Germany | 3 |
| RTECS | WO8400000 |
| F | 10 |
| TSCA | Yes |
| HazardClass | 8 |
| HS Code | 29350090 |
| Hazardous Substances Data | 63-74-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in mice: 3.8 g/kg (Marshall) |
| Sulfanilamide Usage And Synthesis |
| Product features | Sulfanilamide is an organic sulfur compound structurally similar to p-aminobenzoic acid (PABA) with antibacterial property. Sulfanilamide competes with PABA for the bacterial enzyme dihydropteroate synthase, thereby preventing the incorporation of PABA into dihydrofolic acid, the immediate precursor of folic acid. This leads to an inhibition of bacterial folic acid synthesis and de novo synthesis of purines and pyrimidines, ultimately resulting in cell growth arrest and cell death.Without it, bacteria cannot replicate.
|
| Chemical properties | White granular or crystalline powder, odorless. Slightly bitter taste. Slightly soluble in water, ethanol, methanol, ether and acetone, soluble in boiling water, glycerol, hydrochloric acid, sodium hydroxide and potassium hydroxide solution, insoluble in chloroform, ether, benzene, petroleum ether. |
| Uses |
|
| Production methods | There are several methods for their preparation. 1. Acetyl aniline used as raw material The acetanilide reacts with chlorosulfonic acid at 40~50 ℃, and then cooled slowly, added to water for acid decomposition, while precipitation, dried and filtered to give acetaminophen chloride and ammoniated, ammoniated temperature is controlled at 40~45 ℃, hydrolysis, acidification. 2. Method of mixed diphenyl urea Condensation of aniline and urea is single-phenylurea and diphenyl urea (called mixed urea), and then obtained from chlorosulfonated, amination, hydrolysis, acid precipitation. The reaction procedure is as follows. (1)Condensation condensation of aniline hydrochloride and urea, at a temperature of 101~110 ℃ reaction for 3~4 h, obtaining mixed diphenyl urea. (2)Chlorosulfonated Chlorine acid is pressed into the sulfonated pot, stirring cooling, when the temperature drops below 10 ℃, uniformly added mixed phenyl urea under stirring, the reaction temperature is gradually increased, the addition is completed, at the 46~50 ℃ insulation and mixing for 2 h, cooled to below 10 ℃, added water for acid decomposition. Controlling that the decomposition temperature does not exceed 15 ℃, after the addition of water continued stirring for 20min, then by precipitation, washed with water, obtaining mixed phenylurea chloride. (3)Ammoniated 2% aqueous ammonia is put into ammoniated pan, cooled to 25 ℃, stirring and added into a mixing phenyl urea chloride, control the temperature at 40 ℃, insulation and reaction for 3h, obtaining ammoniated liquid. (4)Hydrolysis and neutralization The amide is heated up to 90 ℃, is added 3% lye, continue to heat 108~112 ℃, hydrolysis for 5 h, and moved in the crystallization pot, added hydrochloric acid to neutralize crystals and the crystals are cooled to 20 ℃, crystallization, filtration, washed with water, dry, products are obtained. |
| Uses | The active metabolite of the antibacterial dye, Sulfamidochrysoidine. Inhibits folic acid synthesis in prokaryotes. Antibacterial. |
| General Description | White powder. pH of 0.5% aqueous solution: 5.8-6.1. |
| Air & Water Reactions | May be unstable if exposed for long periods air and light . Slightly water soluble. |
| Reactivity Profile | Sulfanilamide is an amino acid. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May react with azo and diazo compounds to generate toxic gases. |
| Fire Hazard | Flash point data for Sulfanilamide are not available but Sulfanilamide is probably combustible. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- SULPHANILAMIDE 63-74-1 99%
- Inquiry
- 2024-11-05
-
VIP1年
- Apionex Pharma Pvt Ltd
- Sulfanilamide 98%
- Inquiry
- 2024-03-20
-
VIP1年
- Ishita Drugs And Industries Ltd
- 63-74-1 Sulfanilamide 98%
- Inquiry
- 2024-03-16
-
VIP1年
- Orgamine Chemicals(I) Pvt Ltd
- 63-74-1 Sulfanilamide 98%
- Inquiry
- 2024-03-01
-
VIP1年
- Prashant Dyechem Industries
- Sulfanilamide 63-74-1 98%
- Inquiry
- 2024-02-09
-
VIP1年
- Varahi International
- Sulfanilamide 98%
- Inquiry
- 2024-01-29
-
VIP1年
- Merck Ltd
- 63-74-1 98%
- Inquiry
- 2024-01-17
-
VIP1年
- Lakshmi Farmachem
- Sulfanilamide 98%
- Inquiry
- 2024-01-11
-
VIP1年
- Orgo Chem Pvt Ltd
- Sulfanilamide 63-74-1 99%
- Inquiry
- 2024-01-10
-
VIP1年
- Virchow Groups
- Sulfanilamide 98%
- Inquiry
- 2024-01-08
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
