

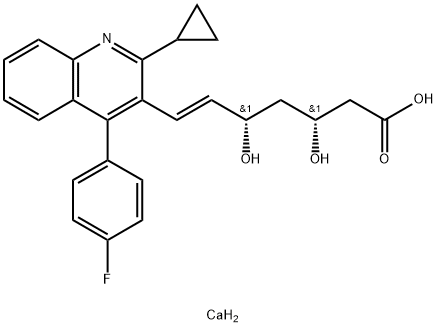
Pitavastatin calcium
| Price | USD7.00 |
| Packge | 1KG |
- Min. Order:1KG
- Supply Ability:1000KG
- Time:2019-07-06
Product Details
- Product Name Pitavastatin calcium
- CAS No. 147526-32-7
- EINECS No.807-641-2
- MFC25H26CaFNO4
- MW463.56
- AppearanceSolidWhite to Off-White
- storage temp. 2-8°C
- Melting point >138oC (dec.)
JD607
| Product Name: | Pitavastatin calcium |
| Synonyms: | Pitavasttin Calcium;Pitavastatin Calcium /NK-104;(+)-Monocalciunbis{(3r,5s,6e)-7-[2-cyclopropyl-4-(4-fluorophenyl-3-quinolyl]-3,5-dihydroxy-6-heptenotate};Pitavastatin calciuM(Livalo);Calcium (3R,5S,E)-7-(2-cyclopropyl-4-(4-fluorophenyl)-quinolin-3-yl)-3,5-dihydroxyhept-6-enoate;Pitavastatin CalciuM DISCONTINUED;(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-6-heptenoic acid,calcium salt (2:1);NK-104 Calcium |
| CAS: | 147526-32-7 |
| MF: | C50H46CaF2N2O8 |
| MW: | 880.98 |
| EINECS: | 1806241-263-5 |
| Product Categories: | Pharmaceuticals;chemical;API;Livalo;Cardiovascular Series;Active Pharmaceutical Ingredients;Inhibitors;Intermediates & Fine Chemicals |
| Mol File: | 147526-32-7.mol |
 |
|
| Pitavastatin calcium Chemical Properties |
| alpha | D20 +23.1° (c = 1.00 in acetonitrile/water) |
| CAS DataBase Reference | 147526-32-7(CAS DataBase Reference) |
| Safety Information |
| Safety Statements | 24/25 |
| Pitavastatin calcium Usage And Synthesis |
| Statin lipid-lowering drugs | Pitavastatin calcium is jointly developed by two companies Nissan Chemical and Kowa Co.it is the first total synthesis HMG-CoA reductase inhibitor, it belongs to statin drugs ,it reduces the ability of the liver to manufacture cholesterol mainly through inhibition of some liver enzymes called HMGCo-A reductase , thus it improves the elevated blood cholesterol levels, it is primarily used for the treatment of hypercholesterolemia and familial hypercholesterolemia patients,its lipid-lowering effect is very good, it is the most potent lipid-lowering drug so far. |
| Pharmacological effects | Inhibition of HMG-CoA reductase: pitavastatin calcium has a strongly antagonistic inhibition effect on HMG-CoA enzyme , IC50 value is 6.8 nmol/L, and its intensity is 24 times of simvastatin, while it is 68 times of fluvastatin. It Hinders the synthesis of cholesterol: the ability to effectively inhibit the process of generating cholesterol in human hepatocytes HepG2 , IC50 value is 5.8nmol/L, and its intensity is 29 times of simvastatin, it is 57 times that of atorvastatin. But pitavastatin calcium inhibition effect on each enzyme in cholesterol generation after generation of mevalonate is very weak. It Increases LDL receptor density: pitavastatin induces the synthesis of LDS receptor mRNA in the ultra-low concentration of 1μmol/L, it can increase the number of LDS receptor mRNA , it results in the increasing of LDL receptor density , thereby it promotes clearance of LDL , so that plasma LDL-plasma total cholesterol concentration and triglyceride concentration decrease. The above information is edited by the Chemicalbook of Tian Ye. |
| Pharmacokinetics | The main parts of its absorption after oral administration are the duodenum and colon,its rate of binding plasma protein in the body is 96%and it is more selectively distributed in the liver after absorption , the drug concentration in body tissues is lower than that in the plasma or the same as that in the plasma . Pitavastatin calcium is mainly metabolized in the liver, kidney, lung, heart, muscle , metabolite concentrations are lower than the concentration of drug prototype, it is excreted through feces,there is also a small amount of drug excretion through urine , total excretion rate is almost 100%. A healthy male adult oral pitavastatin is 0.5~8mg, t1/2 is about 10h,the cmax and AUC of the prototype drug in plasma increase with increasing dose , repeatedly taking does not result in medication savings. |
| Toxicity | Acute toxicity: rats and dogs oral,study its acute toxicity. Pitavastatin calcium median lethal dose on the rats are about male 500~1000mg/kg, female 250~500 mg/kg, dogs lethal dose is about 50~100mg/kg. Long term toxicity: respectively, rats, dogs and monkeys are administered a long-term experiment. From the experimental results, the safe dose of pitavastatin calcium are rats 1 mg/kg · d-1 (6 months), canine 0.3mg/kg · d-1 (12 months), monkey 3mg/kg · d-1 (6 months). No central nervous system, reproductive system, and myocardial dysfunction is observed which is common while taking other statins during administration. Carcinogenicity, mutagenicity: mouse oral 1,12,30, 75mg/kg dose, the incidence of cancer has no significant increase than in the control group .in Chromosome abnormality tests, at the highest concentration of 625μg/ml,the result is positive, but at the same concentration, gene mutation recovery tests, micronucleus test s(in vivo) and UDS test s(in vivo) are negative. |
| Clinical Study | In the therapeutic effect, statins is the first In the lipid-lowering drugs in which pitavastatin effect is very obvious, pitavastatin calcium is a third generation statins anti-hyperlipidemia drug, and Russell atorvastatin (rosuvastatin ) while being called "super statin", is one of the better statins which are current international clinical application of the treatment of hypercholesterolemia, familial hypercholesterolemia , because its clinically effective dose is 1-2mg/day, significantly lower than other marketed statins, with high efficiency, and security features, it has a good tolerability. clinical trail phase I results show that pitavastatin calcium in 1,2,4 mg dose has clinical significance in patients with high blood cholesterol. Results of clinical trail phase Ⅱ determine that the best dosage of the pitavastatin calcium for the treatment of hyperlipidemia is 2mg/d. clinical trail phaseⅢ comparison of experimental results show that the efficacy of pitavastatin calcium on reducing Tc and LDL-c is better than the effect of fluvastatin, and there is no significant difference in safety. Multi-center long-term administration tests carried out in Japan show that the dosage in 1~4mg/d range can effectively control blood lipid levels. The above test results demonstrate the effectiveness of pitavastatin calcium in treatment of hyperlipidemia on clinic. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- 147526-32-7 PITAVASTATIN CALCIUM 95-99%
- Inquiry
- 2024-09-11
-
VIP1年
- Enaltec Labs Pvt Ltd
- 147526-32-7 Pitavastatin calcium 99%
- Inquiry
- 2024-03-21
-
VIP1年
- Zydus Lifesciences Ltd.
- Pitavastatin calcium 98%
- Inquiry
- 2024-01-27
-
VIP1年
- Rivashaa Agrotech Biopharma Pvt. Ltd.
- 147526-32-7 98%
- Inquiry
- 2024-01-25
-
VIP1年
- Solara Active Pharma Sciences Ltd
- 147526-32-7 Pitavastatin calcium 98%
- Inquiry
- 2024-01-23
-
VIP1年
- Nosch Labs Pvt Ltd
- Pitavastatin calcium 147526-32-7 99%
- Inquiry
- 2024-01-09
-
VIP1年
- Hetero Drugs Limited
- Pitavastatin calcium 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Lupin Ltd
- 147526-32-7 99%
- Inquiry
- 2024-01-05
-
VIP1年
- PANCHSHEEL ORGANICS LTD
- PITAVASTATIN CALCIUM 99%
- Inquiry
- 2023-12-29
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
