

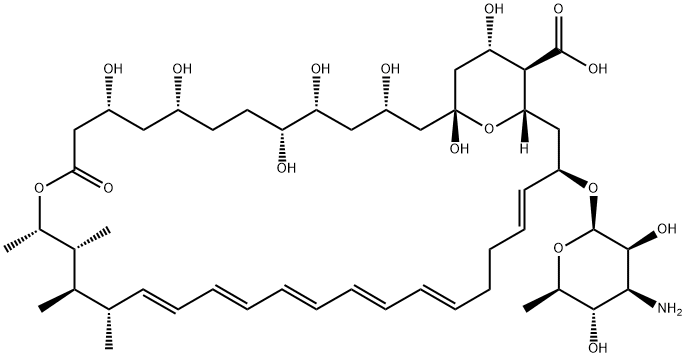
Amphotericin B
| Price | USD10000.00 | USD100.00 |
| Packge | 1KG | 10G |
- Min. Order:10G
- Supply Ability:100KG
- Time:2019-07-06
Product Details
- Product NameAmphotericin B
- CAS No.1397-89-3
- EINECS No.215-742-2
- MFC47H73NO17
- MW924.08
- Appearancepowderyellow
- storage temp. 2-8°C
- Melting point >170°C
- density 1.34
- Water Solubility <0.1 g/100 mL at 21 ºC
- Boiling point 804.34°C (rough estimate)
CR251
| Product Name: | Amphotericin B |
| Synonyms: | ABELCET;AMBISOME;AMPHOTERCIN B;AMPHOTERICIN B;AMPHOTERICIN B SOLUBILIZED;AMPHOTERICIN B, SOLUBLE;AMPHOTERICIN B, STREPTOMYCES NODOSUS;AMPHOTERICIN B, STREPTOMYCES SPECIES |
| CAS: | 1397-89-3 |
| MF: | C47H73NO17 |
| MW: | 924.08 |
| EINECS: | 215-742-2 |
| Product Categories: | Miscellaneous Natural Products;Antibiotic Explorer;Intermediates & Fine Chemicals;Pharmaceuticals;AM to AQAntibiotics;MLS;A;Alphabetic;Chemical Structure;Antibiotics A to;Antibiotics A-FAntibiotics;AntibioticsAntibiotics;AntifungalAntibiotics;Cell Culture;Chemical Structure Class;Interferes with Cell Membrane Permeability (Ionophores)Antibiotics;Mechanism of Action;Polyenes;Reagents and Supplements;Spectrum of Activity;Antifungal;Carbohydrates & Derivatives;Chiral Reagents;ABELCET;antibiotic;Inhibitors |
| Mol File: | 1397-89-3.mol |
 |
|
| Amphotericin B Chemical Properties |
| Melting point | >170°C |
| alpha | D24 +333° (acidic DMF); -33.6° (0.1N methanolic HCl) |
| density | 1.34 |
| storage temp. | 2-8°C |
| solubility | sterile water: 20 mg/mL as a stock solution. Stock solutions should be stored at −20?#x00b0;C. Stable at 37?#x00b0;C for 3 days. |
| form | powder |
| color | yellow |
| Water Solubility | <0.1 g/100 mL at 21 ºC |
| Sensitive | Moisture & Light Sensitive |
| Merck | 13,590 |
| Stability: | Stable, but may be light sensitive. Incompatible with strong oxidizing agents. |
| InChIKey | APKFDSVGJQXUKY-INPOYWNPSA-N |
| CAS DataBase Reference | 1397-89-3(CAS DataBase Reference) |
| EPA Substance Registry System | Amphotericin B(1397-89-3) |
| Safety Information |
| Hazard Codes | C,Xi,Xn,T |
| Risk Statements | 36/37/38-22-40-23/24/25 |
| Safety Statements | 26-36/37/39-45-36-60-37 |
| RIDADR | UN 1759 8/PG 3 |
| WGK Germany | 3 |
| RTECS | BU2625000 |
| F | 8-10-21 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Hazardous Substances Data | 1397-89-3(Hazardous Substances Data) |
| Amphotericin B Usage And Synthesis |
| Polyene antifungal antibiotics | Amphotericin B is a kind of polyene antifungal antibiotics isolated from the culture medium of the Streptomyces (Streptomycesnodosus) which is same substance as the “Lushanmycin” isolated from the actinomyces in the soil of LuShan in JiangXi province of China in 1974. It has significant inhibitory effect on the Cryptococcus neoformans, dermatitis budding yeast, histoplasma capsulatum, sporothrix schenkii, white candida and several kinds of nocardia. The MIC is generally ranged from 0.2 to 0.5 μg/ml. The molecular composition of amphotericin B contains 7 pairs of macrocyclic lactone of conjugated double bond as the ligands and the deoxidized amino hexose of mycosamine as the sugar groups which are connected with the glycosidic bonds. Owing to the existence of both an amino group and carboxyl group in the structure, it is a kind of amphiprotic substance. Its appearance is yellow or orange yellow powder; it is odorless or almost odorless, tasteless and hygroscopic. It is vulnerable to damage and lose function in the sunshine. It is gradually decomposed at over 170 °C and is also not stable at 37 °C. It is soluble in dimethyl sulfoxide (DMSO), slightly soluble in dimethylformamide, extremely slightly soluble in methanol, but insoluble in water, ethanol, and chloroform or ether. It can form salt in either neutral or acidic medium with increased water solubility but a decreased antibacterial activity. Its de-oxidation cholic acid salt complex is light yellow powder and can form gel-like solution in water for injection usage. This product can be stably subject to long-term storage in dry, dark, cold conditions. However, its aqueous solution shouldn’t be maintained at room temperature for more than 24 h, and can also only stored under 4 °C for only one week. Under pH6.0~7.5, it has the strongest antimicrobial effect which decreases at low pH. Its antibacterial mechanism is by combining with the ergosterol on the fungal cell membrane, causing the damage of the membrane and increasing its permeability which further causes the release of cellular substances, therefore resulting in cell death. It is ineffective in treating bacteria due to the lack of ergosterol composition on the bacteria cell membrane. This product is not easy to be absorbed through oral administration and its plasma concentration can be maintained for more than 24 hours after intravenous injection drop wise. It is not easy to penetrate through the blood-brain barrier. Amphotericin B is effective in treating almost all the kinds of fungi with a low incidence of drug-resistant strains. Its price is also relative low which makes it still exhibit high practical value even after its clinical application for more than 40 years. However, the obvious renal toxicity as well as related infusion toxicity (such as fever, chills, nausea, etc.) has greatly restricted amphotericin B rom being widely applied. To reduce the adverse reactions, it has been recently developed in abroad of three types of amphotericin B liposome formulations: amphotericin B liposome, amphotericin B liposome complexes (ABLC) and amphotericin B colloidal dispersion agent (ABCD). Research data have shown that the three kinds of drug formulations have significantly reduced toxicity of amphotericin B while guaranteeing its antifungal activity. In especial, they significantly reduced the incidence of renal toxicity so that can improve the patient's tolerance while guarantee the curative effect of the drug. |
| Pharmacokinetic | Its gastrointestinal absorption is poor and unstable. During the initiation of treatment, intravenous drop 1~5 mg of amphotericin B every day, then gradually increase to 0.65 mg/kg every day after which the blood peak concentration reaches 2~4 mg/L. Its blood clearance half-life is about 24 hours with the protein binding rate being 91%~95%. This drug concentration of this product in the pleural effusion, ascites and synovial cavity liquid is usually lower than half of the blood drug concentration at the same period, and the drug concentration in the bronchial secretions is also low. This product has the highest drug concentration in the renal tissues, followed by liver, spleen, adrenal gland, lung, thyroid, heart, skeletal muscle, and pancreas, etc. This product is slowly excreted in the body through the kidneys with about 2% to 5% daily dosage being excreted in the form of prototype. Its excreted amount through urine within 7 days can account the 70% of the administrated amount. After stopping taking this drug, the drug excretion through urine will continue last for at least seven weeks with increased drug excretion amount in alkaline urine. This product is not easy to be cleaned through dialysis. The above information is edited by the chemicalbook of Dai Xiongfeng. |
| Indications | Amphotericin B is the first-choice drug for treating deep fungus infection with a broad antifungal spectrum. It has inhibitory effect on cryptococcus, coccidioidomyces immitis, candida albicans, and blastomyces with exhibiting bactericidal effect at high concentration, and therefore is a kind of drugs being effective in treating deep fungus infection. Major clinical indications are as follows: 1. It can be used for the treatment of cryptococcosis, North American blastomycosis, disseminated candidiasis, coccidioidomycosis, and histoplasmosis. 2. It can be used for the treatment of mucormycosis caused by the rhizopus genera, absidia, endomycopsis and the basidiobulus sp. 3. It can be used for the treatment of sporotrichum disease caused by schenker sporotrichosis. 4. It can be used for the treatment of aspergillosis caused by the Aspergillus fumigatus. 5. External preparation is suitable for treating chromomycosis, fungal infections infection after burning, respiratory tract candida, Aspergillus or cryptococcus infection, and fungal corneal ulcer. |
| Side effects | 1. During the process of vein drip or after intravenous drip, adverse reactions include chills, fever, severe headache, loss of appetite, nausea, vomiting, and sometimes reduced in blood pressure, dizziness, etc. 2. It can occur for almost all the patients of different degree of renal function impairment during the course of treatment. It can appear in the urine of red blood cells, white blood cells, proteins, and tube type, and increased blood urea nitrogen and creatinine, decreased creatinine clearance rate. It can also cause renal tubular acidosis. 3. The hypokalemia which is due to large amounts of potassium ions discharging through urine. 4. System blood toxicity reaction with normal red blood anemia, sometimes also including leukopenia or thrombocytopenia. 5. Liver toxicity which is relatively rare but can cause liver cell necrosis and also sometimes cause acute liver function failure. 6. The cardiovascular system reaction such as cardiac arrest or ventricular fibrillation during rapid vein drip. In addition, the electrolyte disorder caused by amphotericin B may also lead to the occurrence of arrhythmia. This product can easily lead to thrombophlebitis upon vein drip. 7. Nervous system toxicity reaction: intrathecal injection of this product can cause severe headaches, fever, vomiting, neck stiffness, lower limb pain and urinary retention with paraplegia of lower limb in severe cases. 8. Allergic reactions such as anaphylactic shock, rash occasionally occur. |
| Drug interactions | 1, Adrenal cortical hormone, such drugs can be used in combination for controlling the adverse reactions of amphotericin B, but it is generally not recommended to applied both drugs at the same time for that it can amplify the symptoms of amphotericin B induced hypokalemia. If it is necessary to apply them at the same time, it is recommended to apply adrenal cortical hormone at its minimum dose and with the shortest period of treatment. Moreover, monitoring the potassium concentration and heart function of the patients is also demanded. 2, Digitalis glycosides, hypokalemia caused by this product can strengthen potential digitalis toxicity. Simultaneous application should subject to the close monitor of the blood potassium concentration and heart function. 3, Fluorine cytosine has synergetic effect with amphotericin B, but this product can increase the intake of the former one and do harm to it excretion through renal, and thus further amplifying the toxic effects of fluoride cytosine. 4. This product has antagonist effect in vitro with pyrrole antifungal drugs such as ketoconazolen, fluconazole and itraconazole. 5, Nephrotoxicity drugs such as aminoglycoside, antitumor drugs, capreomycin, polymyxin class and vancomycin can have their nephrotoxicity enhanced when used in combination with this drug. 6, Bone marrow inhibitor and radiotherapy can aggravate the anemia o patients, and therefore should be applied with reduced amount when used in combination with amphotericin B. 7, the hypokalemia induced by this product can strengthen the effect of neuromuscular blockers. Therefore, the monitor of potassium concentration is demanded when these two kinds of drugs are used in combination. 8, application of urine alkaline medicine can enhance the excretion of this product and prevent or reduce of possibility of the occurrence o renal tubular acidosis. |
| Amphotericin B liposome | The effective component in amphotericin B liposome is amphotericin B. Its cholesterol content can enhance the stability of the drug to retain the amphotericin B content in the hydrophobic layer as much as possible and reduces its binding to the cholesterol of human cell membrane while enhances its binding to the ergosterol of fungal cells membrane, and thus playing the maximal bactericidal ability of amphotericin B. Its mechanism of action is similar to amphotericin B which is through binding to the fungal cell membrane sterol (mainly ergosterol), further increasing the membrane permeability, and causing release of important material inside the cells (e.g., potassium, amino acids and nucleotides) which lead to the deaths of fungal cells. Amphotericin B liposome mainly contains three dosage forms: (1) amphotericin B lipid complexes (crosslink of amphotericin B with liposome) (2) amphotericin B liposome (use liposome to wrap the amphotericin B inside). (3) amphotericin B colloidal dispersion (warp the mixture between cholesterol sulfates with the same amount of amphotericin B). Inside the body, the preparations of these lipids are mostly distributed in the reticular endothelial tissue (such as the liver, spleen and lung tissue), reducing the drug distribution in the kidney tissues, and thereby reducing the nephrotoxicity of amphotericin B. In addition, blood creatinine levels rise and hypokalemia also becomes rare; the incidence of toxic effect related to the intravenous drip is also significantly lower than that of amphotericin B. Therefore, amphotericin B liposome keeps the high antibacterial activity of amphotericin B while reducing their toxicity. The product has good antifungal activity against cryptococcus neoformans, candida albicans, candida tropical, yeast, aspergillus, coccidioidomyces immitis, Histoplasma, blastomyces demantitidis and Brazil blastomyces. However, it has no activity against bacteria, Rickettsia and virus. Part of the aspergillus has drug resistance with Skin and hair ringworms are mostly resistant to it. |
| Chemical properties | It is pale yellow to orange needle crystal or powder. It is insoluble in water, ethanol, but soluble in acidic DMF, DMSO, and slightly soluble in DMF, acidic or alkaline water-containing lower alcohol. It is almost odorless, almost tasteless, and easy to be damaged by the light, heat and acid. |
| Uses | 1. The product has strong inhibitory effect on various kinds of fungal infection, such as Cryptococcus neoformans, dermatitis budding yeast, histoplasma capsulatum, sporothrix schenkii, white candida, mucor sp and Coccidioides immitis. The goods are the primary choice of drugs for treating deep fungal disease. 2. It is a kind of polyene antifungal drugs. This drug binds to the sterol located on the fungal cell membranes sterol, disrupting the membrane permeability, causing the leakage of bacteria intracellular potassium ion, nucleotides, amino acids and therefore playing bactericidal effect by disrupting the normal metabolism. |
| Production methods | Streptomyces strains for is used as the target strain. It is subject to submerged aerobic fermentation in the liquid medium containing both carbohydrates and organic nitrogen source. Upon reaching a certain titer units, extract the amphotericin from the fermented liquid. Amphotericin contains two ingredients, A and B with composition of A having a small toxicity and weak antifungal effect which is not suitable for clinical purposes; Composition B has a strong effect, called as amphotericin B. |
| Category | Toxic substances. |
| Toxicity grading | Highly Poisonous. |
| Acute toxicity | Vein-LD50 in rats: 11.3 mg/kg; Abdominal cavity-LD50 in mice: 27.74 mg/kg. |
| Chemical Properties | Crystalline Yellow Solid |
| Uses | Polypeptide antibiotic active against gram positive bacteria. Antifungal. |
| Uses | Amphotericin B is heptaene polyene antifungal originally discovered as a metabolite of Streptomyces nodosus in 1956. Amphotericin B acts by binding sterols in the cell membrane leading to the formation of transmembrane channels and subsequent ion leakage. Amphotericin B is poorly water soluble so has been developed for therapeutic use as a complex with desoxylate or in liposomes to improve bioavailability. Amphotericin B is widely used as a research reagent in diverse applications with over 15,000 literature citations. |
| Definition | ChEBI: A macrolide antibiotic used to treat potentially life-threatening fungal infections. |
| Flammability and hazard characteristics | Combustible; Heating yields toxic nitrogen oxides smoke. |
| Storage and transport properties | Warehouse: ventilation, dry and low temperature. |
| Fire extinguishing agent | Dry powder, foam, sand, carbon dioxide. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- AKASH PHARMA EXPORTS
- Amphotericin 99%
- Inquiry
- 2024-05-16
-
VIP1年
- Asence Pharma Pvt Ltd
- Amphotericin B 99%
- Inquiry
- 2024-03-29
-
VIP1年
- Century Pharmaceuticals Ltd
- Amphotericin B 1397-89-3 99%
- Inquiry
- 2024-03-07
-
VIP1年
- BDR Pharmaceuticals International Pvt Ltd
- Amphotericin B 98%
- Inquiry
- 2024-02-24
-
VIP1年
- PROTECH TELELINKS
- 1397-89-3 98%
- Inquiry
- 2024-02-09
-
VIP1年
- Ralington Pharma
- 1397-89-3 Amphotericin B 98%
- Inquiry
- 2024-02-05
-
VIP1年
- Synbiotics Limited
- Amphotericin B 1397-89-3 98%
- Inquiry
- 2024-01-11
-
VIP1年
- Bajaj Healthcare Ltd
- Amphotericin B 98%
- Inquiry
- 2024-01-08
-
VIP1年
- Varanous Labs Pvt Ltd
- 1397-89-3 amphotericin b 98%
- Inquiry
- 2023-12-26
-
VIP1年
- Varanous Labs Pvt Ltd
- 1397-89-3 98%
- Inquiry
- 2023-12-26
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
