

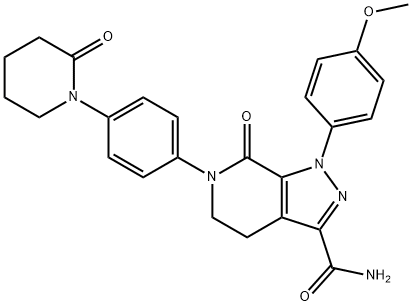
Apixaban
| Price | USD100.00 |
| Packge | 25KG |
- Min. Order:1KG
- Supply Ability:1000KG
- Time:2019-07-06
Product Details
- Product NameApixaban
- CAS No.503612-47-3
- EINECS No.1308068-626-2
- MFC25H25N5O4
- MW459.5
- AppearanceSolidWhite to Off-White
- density 1.42
- Boiling point 770.5±60.0 °C(Predicted)
- Melting point 235-238°C
- storage temp. Refrigerator
CR076
| Apixaban Basic information |
| Product Name: | Apixaban |
| Synonyms: | Apixaban;Apixaban, BMS 562247-01;1H-Pyrazolo[3,4-c]pyridine-3-carboxamide, 4,5,6,7-tetrahydro-1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl]-;1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide;BMS 562247-01;1-(4-Methoxy-phenyl)-7-oxo-6-[4-(2-oxo-piperidin-1-yl)-phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid aMide;1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4, 5-dihydropyrazolo[3,4-c]pyridine-3-carboxaMide;Nilotinib and its interMediate |
| CAS: | 503612-47-3 |
| MF: | C25H25N5O4 |
| MW: | 459.504 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Inhibitor;Inhibitors |
| Mol File: | 503612-47-3.mol |
 |
|
| Apixaban Chemical Properties |
| density | 1.42 |
| Safety Information |
| Apixaban Usage And Synthesis |
| Indications and Usage | Apixaban is a new form of oral anticoagulant drug developed by Bristol Myers Squibb and Pfizer. It is a new form of oral Xa factor inhibitor, and its commercial name is Eliquis. Apixaban is used to treat adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolism (VTE) |
| Mechanisms of Action | Apixaban is an oral selective activated Xa factor inhibitor and can prevent thrombin generation and thrombosis. |
| Clinical Research | Apixaban is the third new oral anticoagulant to go on sale, following dabigatran and rivaroxaban, and it has already been approved in Europe for preventing venous thromboembolism in patients undergoing elective hip or knee replacement surgery. Out of these three oral anticoagulants approved in Europe, compared to the current standard preventative treatment against venous thromboembolism, enoxaparin, rivaroxaban excelled in the record experiment, and apixaban excelled in the advance experiment. Rivaroxaban’s curative effects were slightly superior, but it caused more severe bleeding than apixaban. Researchers attributed these differences to medication time, as rivaroxaban was taken 6-8 hours after surgery in the record experiment, while apixaban was used 18 hours after surgery in the advance experiment. These drugs have better curative effect when used closer to time of surgery, but also have an increased bleeding risk. Clinical research showed that compared to a daily subdermal injection of 40mg enoxaparin, 2 oral 2.5mg dosages of apixaban had better preventative effects against venous thromboembolism following hip or knee replacement surgery and did not increase bleeding risk. |
| Drug Interactions | 1.A double inhibitor of strong CYP3A4 and P-gp increases apixaban’s blood levels: decrease Eliquis dosage to 2.5mg or avoid simultaneous usage. 2.A inductor of strong CYP3A4 and P-gp can decrease apixaban’s blood levels: avoid simultaneous usage. |
| Warnings and Precautions |
|
| Uses | Apixaban is a highly selective, reversible inhibitor of Factor Xa with Ki of 0.08 nM and 0.17 nM in human and rabbit, respectively. |
| Definition | ChEBI: A pyrazolopyridine that is 7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide substituted at position 1 by a 4-methoxyphenyl group and at position 6 by a 4-(2-oxopiperidin-1-yl)phenyl group. It is used for the prevention and treatment of thromboembolic diseases. |
| References | https://en.wikipedia.org/wiki/Apixaban https://www.drugbank.ca/drugs/DB06605 |
| Apixaban Preparation Products And Raw materials |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- 503612-47-3 95-99%
- Inquiry
- 2024-09-11
-
VIP1年
- Molsyns Research
- 503612-47-3 98%
- Inquiry
- 2024-04-01
-
VIP1年
- Symed Laboratories Ltd
- 503612-47-3 Apixaban 98%
- Inquiry
- 2024-03-21
-
VIP1年
- Amoli Organics Pvt Ltd
- 503612-47-3 98%
- Inquiry
- 2024-03-20
-
VIP1年
- ASolution Pharmaceuticals Pvt Ltd
- 503612-47-3 Apixaban 98%
- Inquiry
- 2024-03-12
-
VIP1年
- Srini Pharmaceuticals Pvt Ltd
- Apixaban 503612-47-3 98%
- Inquiry
- 2024-03-04
-
VIP1年
- Cohance Lifesciences (Previously RA Chem Pharma Ltd)
- Apixaban 98%
- Inquiry
- 2024-02-28
-
VIP1年
- BDR Pharmaceuticals International Pvt Ltd
- 503612-47-3 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Globela Industry Pvt Ltd
- 503612-47-3 Apixaban 98%
- Inquiry
- 2024-02-22
-
VIP0年
- Glyra Health Care Pvt Ltd
- Apixaban 503612-47-3 99%
- Inquiry
- 2024-02-21
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
