| Important pharmaceutical intermediates |
Piperazine is an important pharmaceutical intermediate, is mainly used for the production of anthelmintic piperazine phosphate, piperazine citrate and fluphenazine, strong pain, rifampicin, adipic acid piperazine, piperazine guanidine methyl tetracycline, quinoline piperazine phosphate, piperazine thiazole nitrate, enoxacin, hydroxyzine hydrochloride, trifluoperazine, diethylcarbamazine citrate, cinnarizine, flunarizine, decloxizine strong carbamazepine, prednisolone sodium phosphate, dexamethasone sodium phosphate, PPA, norfloxacin, ciprofloxacin, easy to cough piperazine, a piperazine Lee vancomycin, trimethoprim-triazine and other drugs. It is Also used for the production of surfactants products such as wetting agents, emulsifying agents,and dispersing agents ,and the production of plastic additives such as antioxidants, preservatives, stabilizers and rubber additives. It is derived from Dichloroethane by alcohol solution of ammonia.
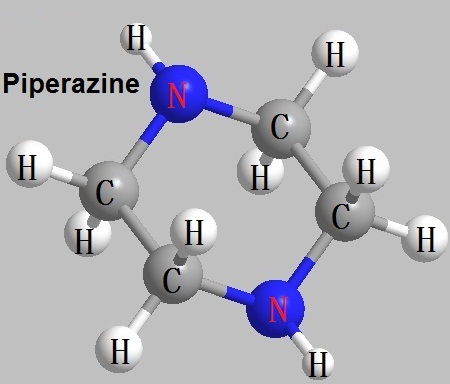
Figure 1 The structural formula of piperazine. |
| Anthelmintic |
Piperazine is a safe and effective anti-roundworm and pinworm medicine, China's "Veterinary Pharmacopoeia" collection contains as piperazine citrate and phosphoric acid piperazine, the product can make roundworm muscle paralysis, so insects body can not be attached to the host intestinal wall and excreted with the feces. Piperazine mechanism is that it is capable of blocking cholinergic receptors at the neuromuscular junction, thereby preventing excitatory effects transmission of nerve impulses and acetylcholine. No excitement before the worm paralysis phenomenon, it is more secure. After oral ,it is rapidly absorbed by the gastrointestinal tract, it is metabolized in the body part, the rest is excreted by the kidneys. Its various salts in solution have become piperazine hexahydrate, absorption and excretion are basically the same, but the rate of excretion is of large individual differences. It is Clinically used for intestinal ascariasis, incomplete intestinal obstruction due to roundworm or early biliary ascariasis , it is also used for pinworm infection.
Various salts of piperazine ( more stable than piperazine) belong to low toxicity, efficient drive roundworm medicine, in addition to roundworms, also having impact on Oesophagostomum, pointed filaria , it has been widely used in veterinary clinic. Deworming effect of piperazine, is anticholinergic-like effect on neuromuscular joints in roundworms, thereby blocking the transmission of nerve impulses; while the worms function of producing succinic acid is also blocked, resulting in parasite paralysis, losing the ability to host attached to the intestinal wall, and by peristalsis is excreted with the feces. Piperazine and its salts can be quickly absorbed from the gastrointestinal tract, part of the metabolism in tissues (30% to 40%) the rest of the urinary excretion.
Piperazine has excellent insecticide effect on Ascaris and Oesophagostomum ,it is a common drive roundworm medication, once having a 100% dispel effect. But it has limited role in the larvae , the medication should be 1 times more after 2 months.
The above information is edited by the chemicalbook of Tian Ye. |
| Chemical Properties |
Piperazine is a white crystalline, m.p.108~110 ℃, b.p.145~146 ℃, hygroscopic, soluble in water and ethanol, insoluble in ether, the aqueous alkaline reaction. piperazine is Usually as a commodity piperazine hexahydrate.Piperazine hexahydrate(CAS [142-63-2]) is a white crystalline, hygroscopic, m.p.44~45 ℃, b.p.145~156 ℃, soluble in water and ethanol, insoluble in ether. |
| Uses |
It is mainly used for the production of anthelmintic piperazine phosphate, piperazine citrate.Piperazine hexahydrate is dissolved in ethanol, piperazine acetic acid is precipitated by adding glacial acetic acid stirring and cooling , it is used in the synthesis of steroids prednisolone sodium phosphate. Piperazine hexahydrate reacts with acetic anhydride to prepare intermediate acetyl piperazine of anthelmintic piperazine nitro thiazole . |
| production method |
The preparation method is using chloroethanol as raw material, obtainning after ammonification, cyclization. The process is making chlorine ethanol and ammonium hydroxide heated in an autoclave to ammonia pressure of 441 kPa, the reaction is stirred for 5h, recover excess ammonium hydroxide aqueous solution, obtain amino alcohol. Quantitative hydrochloric acid is added with stirring, concentrate under reduced pressure to a semi-paste ethanolamine hydrochloride concentrate , heat and get rid of water to 150 ℃, add prior molten paraffin good, and then continue to heat up to 260 ℃, holding (260 ± 2) ℃ for 12h , filtere to remove the paraffin, to obtain hydrochloride piperazine. Piperazine hydrochloride, solid sodium hydroxide, water are added to the reaction vessel and stirred 1.5h, excrete ammonia, and then heat to 130~140 ℃, distill piperazine hexahydrate solution, the residue is filtered, PIPERAZINE HEXAHYDRATE solution is chilled to 8 ℃, centrifugal rejection filter piperazine hexahydrate, piperazine is obtained by piperazine hexahydrate dehydration. Reaction equation as: ClCH2CH2OH + NH4OH → NH2CH2CH2OH · HCl + H2O |
| Category |
corrosive materials |
| Toxicity grading |
poisoning |
| Acute oral toxicity |
rat LD50: 1900 mg/kg; Oral-Mouse LD50: 600 mg/kg |
| Data Skin irritation |
rabbit 500 mg Mild; Eyes-rabbit 0.25 mg/24 hours of severe |
| Hazardous characteristics |
explosive when mixed with air |
| Flammability and hazardous characteristics |
Combustible; decomposition of toxic nitric oxide gas in case of thermal |
| Storage characteristics |
Treasury ventilation low-temperature drying; and stored separately from acid |
| Chemical Properties |
Solid |
| Extinguishing agent |
Water spray, dry powder, carbon dioxide, alcohol-resistant foam |
| Uses |
Labelled Piperazine |
| Uses |
keratolytic, antiseborheic |
| Definition |
ChEBI: An azacycloalkane that consists of a six-membered ring containing two nitrogen atoms at opposite positions. |
| Professional standards |
TWA 1 mg/m³; STEL 5 mg/m |
| General Description |
Needle-like white or colorless crystals. Shipped as a solid or suspended in a liquid medium. Very corrosive to skin, eyes and mucous membranes. Solid turns dark when exposed to light. Flash point 190°F. Used as a corrosion inhibitor and as an insecticide. |
| Air & Water Reactions |
Flammable. Absorbs water and carbon dioxide from air. Soluble in water. |
| Reactivity Profile |
1,4-Diazacyclohexane neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Absorbs carbon dioxide from the air, which can cause dry crystals to seem to melt. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. 1,4-Diazacyclohexane is sensitive to light; 1,4-Diazacyclohexane absorbs water and carbon dioxide from air. 1,4-Diazacyclohexane may be corrosive to aluminum, magnesium and zinc. . |
| Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
| Fire Hazard |
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |





