

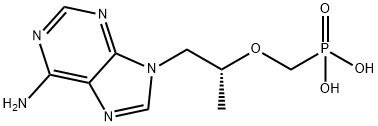
Tenofovir
| Price | USD130.00 |
| Packge | 1G |
- Min. Order:1G
- Supply Ability:1kg
- Time:2019-07-06
Product Details
- Product NameTenofovir
- CAS No.147127-20-6
- EINECS No.240-723-0
- MFC9H14N5O4P
- MW287.21
- Appearancepowderwhite to beige
- Melting point 276-280°C
- Water Solubility 13.4 mg/mL (25 ºC)
- storage temp. -20°C
- Boiling point 616.1±65.0 °C(Predicted)
- density 1.79±0.1 g/cm3(Predicted)
CR29
| Tenofovir Basic information |
| Product Name: | Tenofovir |
| Synonyms: | 1-(6-aminopurin-9-yl)propan-2-yloxymethylphosphonic acid;D,L-TENOFOVIR;TENOFOVIR;(R)-9-(2-PHOSPHONYLMETHOXYPROPYL)-ADENINE;(R)-9-(2-Phosphonomethoxypropyl)Adenine;Tenofovir(ForResearchOnly);(R)-PMPA;[[(1R)-2(6-Amino-9H-purin-9-yl)-1-methylethoxy]methyl]phosphonic Acid |
| CAS: | 147127-20-6 |
| MF: | C9H14N5O4P |
| MW: | 287.21 |
| EINECS: | 1308068-626-2 |
| Product Categories: | Purine;ACTIVE PHARMACEUTICAL INGREDIENTS;Nucleotides and Nucleosides;Bases & Related Reagents;Intermediates & Fine Chemicals;Nucleotides;Pharmaceuticals;Phosphorylating and Phosphitylating Agents;Inhibitors |
| Mol File: | 147127-20-6.mol |
 |
|
| Tenofovir Chemical Properties |
| Melting point | 276-280°C |
| alpha | D +21° (c = 1 in 0.1M HCl) |
| storage temp. | Store at -20°C |
| Water Solubility | 13.4 mg/mL (25 ºC) |
| Merck | 14,9146 |
| CAS DataBase Reference | 147127-20-6(CAS DataBase Reference) |
| Safety Information |
| RTECS | SZ6563600 |
| Tenofovir Usage And Synthesis |
| Indications and Usage | Tenofovir disoproxil (Viread) is the first nucleotide analogue approved by the American FDA to treat HIV-1 infections. Tenofovir disoproxil is a drug used in the AIDS cocktail treatment method, and research shows that it has the ability to increase monkeys’ immunity to immunodeficiency viruses (similar to the human AIDS virus). Tenofovir disoproxil is used in combination with other reverse transcriptase inhibitors to treat HIV-1 infections and hepatitis B. |
| Mechanisms of Action | Tenofovir disoproxil is an acyclic nucleoside antivirus drug and has an inhibiting effect on HBV multi-enzyme complexes and HIV reverse transcriptase. Its active content tenofovir phosphonate directly competitively binds to natural deoxyribose substrate to inhibit the virus multi-enzyme complex and inserts itself into the DNA to end the nucleotide chain. Tenofovir disoproxil is barely absorbed by the gastrointestinal duct, so it undergoes esterification and ionization to become tenofovir ester fumarate. Tenofovir is soluble in water and can be quickly absorbed and decomposed into the active substance tenofovir, which then transforms into the active metabolite tenofovir phosphonate. As this drug is not metabolized by the CYP450 enzyme system, it has a very low chance of drug interactions caused by this enzyme. |
| Pharmacokinetics | Tenofovir disoproxil reaches peak blood concentration 1-2 hours after intake. Tenofovir disoproxil’s bioavailability increases by about 40% when taken with food. The intracellular half-life of tenofovir phosphonate is about 10 hours, so doses can be taken once daily. This drug is mainly filtered through renal glomeruli and excreted through the renal tubule transport system, with 70-80% excreted in its original form through urine. |
| Adverse Effects |
|
| Chemical Properties | White Crystalline Solid |
| Uses | Tenofovir is a drug used for the treatment of chronic heptatitis B as well as prevention and treatment of HIV/AIDS. It is a kind of nucleotide analog, acting as the reverse-transcriptase inhibitor (NtRTI). It inhibits the activity of HIV reverse transcriptase through competing with the natural substrate deoxyadenosine 5’-triphosphate, causing the termination of DNA chain. |
| Definition | ChEBI: A member of the class of phosphonic acids that is methylphosphonic acid in which one of the methyl hydrogens is replaced by a [(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy group. An inhibitor of HIV-1 reverse transcriptase, the bis(isopropyloxycarbonyloxy ethyl) ester (disoproxil ester) prodrug is used as the fumaric acid salt in combination therapy for the treatment of HIV infection. |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- Aspen Biopharma Labs Pvt Ltd
- 147127-20-6 Tenofovir 98%
- Inquiry
- 2024-03-16
-
VIP1年
- Vishrudh laboratories pvt ltd
- Tenofovir 147127-20-6 98%
- Inquiry
- 2024-03-01
-
VIP1年
- Arene Lifesciences Limited
- Tenofovir 99%
- Inquiry
- 2024-02-05
-
VIP1年
- Raising Sun Pharma
- 147127-20-6 98%
- Inquiry
- 2024-02-03
-
VIP1年
- VYAS Chemicals
- 147127-20-6 Tenofovir 98%
- Inquiry
- 2024-01-19
-
VIP1年
- DSN Labs
- Tenofovir 147127-20-6 98%
- Inquiry
- 2024-01-12
-
VIP1年
- CNS CHEMICALS
- Tenofovir 98%
- Inquiry
- 2024-01-03
-
VIP1年
- Dodhia Group
- Tenofovir 99%
- Inquiry
- 2024-01-03
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
