

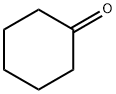
Cyclohexanone
| Price | USD2.00 | USD1.00 |
| Packge | 1KG | 5KG |
- Min. Order:1000KG
- Supply Ability:100MT/Monthly or Customized
- Time:2019-07-06
Product Details
- Product NameCyclohexanone
- CAS No.108-94-1
- EINECS No.203-631-1
- MFC6H10O
- MW98.14
- InChIKeyJHIVVAPYMSGYDF-UHFFFAOYSA-N
- AppearanceLiquidAPHA: ≤10
- Melting point -47 °C (lit.)
- density 0.947 g/mL at 25 °C (lit.)
- Water Solubility 150 g/L (10 ºC)
- Boiling point 155 °C (lit.)
- storage temp. Store at +5°C to +30°C.
SP012
| Cyclohexanone Basic information |
| Product Name: | Cyclohexanone |
| Synonyms: | Anon;caswellno270;Cicloesanone;Cykloheksanon;cykloheksanon(polish);epapesticidechemicalcode025902;Hexanon;Hytrol O |
| CAS: | 108-94-1 |
| MF: | C6H10O |
| MW: | 98.14 |
| EINECS: | 203-631-1 |
| Product Categories: | Intermediates of Dyes and Pigments;Alphabetical Listings;C-D;Flavors and Fragrances;Alpha Sort;C;CAlphabetic;CO - CZ;Volatiles/ Semivolatiles;Analytical Reagents for General Use;C-D, Puriss p.a.;Puriss p.a.;ACS GradeCarbonyl Compounds;C3 to C6;Essential Chemicals;Ketones;Routine Reagents;Analytical/Chromatography;Auxiliaries for ISE;Ion Sensor Materials;Reagent Plus;Halogenated Heterocycles ,Indolines ,Indoles ,Indazoles;pharmaceutical intermediate |
| Mol File: | 108-94-1.mol |
 |
|
| Cyclohexanone Chemical Properties |
| Melting point | -47 °C |
| Boiling point | 155 °C(lit.) |
| density | 0.947 g/mL at 25 °C(lit.) |
| vapor density | 3.4 (vs air) |
| vapor pressure | 3.4 mm Hg ( 20 °C) |
| refractive index | n20/D 1.450(lit.) |
| FEMA | 3909 | CYCLOHEXANONE |
| Fp | 116 °F |
| storage temp. | Flammables area |
| solubility | 90g/l |
| pka | 17(at 25℃) |
| form | Liquid |
| color | APHA: ≤10 |
| Relative polarity | 0.281 |
| PH | 7 (70g/l, H2O, 20℃) |
| explosive limit | 1.1%, 100°F |
| Water Solubility | 150 g/L (10 ºC) |
| Merck | 14,2726 |
| BRN | 385735 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 108-94-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexanone(108-94-1) |
| EPA Substance Registry System | Cyclohexanone(108-94-1) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 10-20-41-38-20/21/22 |
| Safety Statements | 25-36/37/39-26 |
| RIDADR | UN 1915 3/PG 3 |
| WGK Germany | 1 |
| RTECS | GW1050000 |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| Hazardous Substances Data | 108-94-1(Hazardous Substances Data) |
| Cyclohexanone Usage And Synthesis |
| Production Method | In the 1940s, the industrial production of cyclohexanone mainly applied hydrogenation of phenol to generate cyclohexanol, followed by dehydrogenation to give cyclohexanone. In the 1960s, with the development of petrochemical industry, the cyclohexane oxidation production method gradually dominated. In 1967, the one step method of phenol hydrogenation, developed by the Netherlands National Mining Company (DSM) was industrialized. This method has short production process, good product quality and high yield, but the raw materials of phenol and catalyst are expensive, so the majority of the industry still adopts the cyclohexane oxidation method. 1. Phenol method takes nickel as a catalyst; first apply hydrogenation of phenol to give cyclohexanol, followed by dehydrogenation to give cyclohexanone using zinc as the catalyst for zinc. 2. Cyclohexane oxidation method uses cyclohexane as the raw material; first apply non-catalyst condition; use oxygen-rich air for oxidation to give cyclohexyl hydroperoxide, followed by decomposition into the mixture of cyclohexanol, cyclohexanone, alcohol and ketone in the presence of tert-butyl chromate catalyst; further apply a series of distillation refinement to get qualified products. Raw material consumption quota: cyclohexane (99.6%) 1040kg / t. 3. Benzene hydrogenation oxidation method; benzene subjects to hydrogenation (with hydrogen) at 120-180 ℃ in the presence of nickel catalyst to generate cyclohexane; cyclohexane has oxidation reaction with air at 150-160 ℃, 0.908MPa to obtain the mixture of cyclohexanol and cyclohexanone; separate them to obtain the cyclohexanone product. Cyclohexanol is dehydrogenated at 350-400 ° C in the presence of a zinc-calcium catalyst to produce cyclohexanone. Raw material consumption quotas: benzene (99.5%) 1144kg / t, hydrogen (97.0%) 1108kg / t, caustic soda (42.0%) 230kg / t. |
| Chemical Properties | colourless liquid |
| Definition | ChEBI: A cyclic ketone that consists of cyclohexane bearing a single oxo substituent. |
| General Description | A colorless to pale yellow liquid with a pleasant odor. Less dense than water . Flash point 111°F. Vapors heavier than air. Used to make nylon, as a chemical reaction medium, and as a solvent. |
| Air & Water Reactions | Flammable. Soluble in water. |
| Reactivity Profile | Cyclohexanone forms an explosive peroxide with H2O2, and reacts vigorously with oxidizing materials (nitric acid). |
| Health Hazard | Inhalation of vapors from hot material can cause narcosis. The liquid may cause dermatitis. |
| Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Cyclohexanone Preparation Products And Raw materials |
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- ARRAKIS INDUSTRIES LLP
- 108-94-1 CYCLOHEXANONE 99%
- Inquiry
- 2024-11-05
-
VIP1年
- SS Reagents and Chemicals
- 108-94-1 Cyclohexanone 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Pallav Chemicals And Solvents Pvt Ltd
- 108-94-1 Cyclohexanone 99% Extrapure 99%
- Inquiry
- 2024-10-24
-
VIP1年
- Nabariya Impex
- Cyclo Hexanone 99%
- Inquiry
- 2024-10-23
-
VIP1年
- Gujarat State Fertilizers And Chemicals Ltd
- Cyclohexanone 108-94-1 98%
- Inquiry
- 2024-02-27
-
VIP1年
- LANXESS India Pvt. Ltd.
- Cyclohexanone 98%
- Inquiry
- 2024-01-09
-
VIP1年
- JSK Chemicals
- 108-94-1 99%
- Inquiry
- 2023-12-22
-
VIP1年
- Choice Chemicals
- Cyclo Hexanone 99%
- Inquiry
- 2023-12-19
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
