

Luliconazole
| Price | USD3000.00 | USD2980.00 | USD2960.00 |
| Packge | 1KG | 5KG | 10KG |
- Min. Order:2KG
- Supply Ability:Monthly 100KG or Customized
- Time:2019-07-06
Product Details
- Product NameLuliconazole
- CAS No.187164-19-8
- EINECS No.247-960-9
- MFC14H9Cl2N3S2
- MW354.27
- AppearanceSolidWhite to Off-White
- Boiling point 499.1±55.0 °C(Predicted)
- density 1.52±0.1 g/cm3(Predicted)
- Melting point 152 °C
- storage temp. Sealed in dry,Store in freezer, under -20°C
SP009
▼
▲
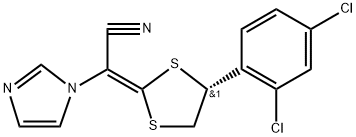
Product Name:
Synonyms:
Luliconazole;(2E)-2-[(4R)-4-(2,4-Dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-imidazol-1-ylacetonitrile;4-(2,4-Dichlorophenyl)-1,3-dithiolan-2-ylidene-1-imidazolylacetonitrile;C13478;Lulicon;NND 502;1H-Imidazole-1-acetonitrile,a-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-,(aE)-;Luliconazole (This product is only available in Japan.)
CAS:
MF:
C14H10Cl2N3S2
MW:
354.28
EINECS:
Product Categories:
Mol File:
▼
▲
▼
▲
▼
▲
▼
▲
Luliconazole Usage And Synthesis
▼
▲
Outline
Luliconazole is a novel topical antifungal imidazole, and is a kind of analogue of lanoconazole. It can interfere with the fungal cell wall synthesis and fungal growth by decreasing levels of ergosterol via inhibiting lanosterol demethylase activity. Besides being used for the treatment of athlete's foot, jock itch and ringworm, it has also been developed for onychomycosis (nail fungus) treatment and has now also entered the clinical stage phase III. This product was originally developed by the Japanese pesticide Corporation (NihonNohyaku Co., Ltd.). In November 2013, the FDA has approved a 1% luliconazole cream for entering into market for topical treatment of interdigital athlete's foot, jock itch and ringworm with the trade name being Luzu and first entered into market in North America. As early as April 2005, luliconazole had been approved to enter into market in Japan under the trade name Lulicon. In January 2010 and June 2012, it was approved for marketing in India and China, respectively.
In 1997, Pesticide Co., Ltd. of Japan has initially get access to the worldwide patent of luliconazole as antifungal agents (WO 1997002821 A2) and have protected in their preparation and application; thereafter, it had also applied for a European patent (EP0839035 A2), Chinese patent (CN 1194582 A) and U.S. Patent (US5900488A). In addition, WO 2007102241, US 8058303, and other patents have also been applied for protection on the drug's pharmaceutical compositions and dosage forms.
In 1997, Pesticide Co., Ltd. of Japan has initially get access to the worldwide patent of luliconazole as antifungal agents (WO 1997002821 A2) and have protected in their preparation and application; thereafter, it had also applied for a European patent (EP0839035 A2), Chinese patent (CN 1194582 A) and U.S. Patent (US5900488A). In addition, WO 2007102241, US 8058303, and other patents have also been applied for protection on the drug's pharmaceutical compositions and dosage forms.
Synthetic Method
The first method is using BH3/THF and a kind of chiral catalyst for stereoselectively reducing the starting material 1 to give the intermediate 2 which yields the corresponding mesylate intermediate (3), substance 3 and intermediate 5 is cyclized into luliconazole in the presence of potassium hydroxide and DMSO; second method is based on using chiral compound 6 as starting materials, 6 undergoes mesylate esterification to yield active intermediate 7,7 undergoes condensation reaction with intermediate 5 to get luliconazole. The synthesis of Intermediate 5 is through putting 2-(1-imidazolyl)-acetonitrile and CS2 into condensation under basic conditions.
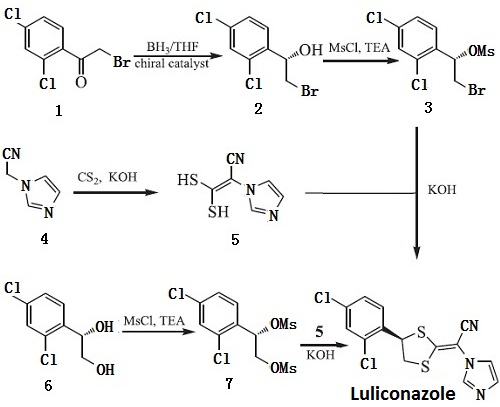
Figure 1 is a synthesis route of luliconazole

Figure 1 is a synthesis route of luliconazole
Application
It can be used for the following fungal infections:
Ringworm: athlete's foot, ringworm, jock itch;
Candida infections: disease fingers erosion, intertrigo; vitiligo.
Ringworm: athlete's foot, ringworm, jock itch;
Candida infections: disease fingers erosion, intertrigo; vitiligo.
Currently, ringworm treatment drugs include two major categories: first, propylene amine drugs, such as terbinafine, Butenafine and naftifine. They exert their bactericidal effects through inhibiting squalene cyclase, causing the lack of ergosterol and accumulation of squalene. The second category of imidazole (imidazoles) drugs: such as miconazole, econazole, clotrimazole, ketoconazole and bifonazole. They are a class of synthetic antifungal agent that can selectively inhibit the lanosterol 14α-demethylation activity of fungal cell, preventing the ergosterol synthesis of cell membrane, changing the cell membrane permeability, and resulting in the loss of important intracellular fungal material and causing fungal death. Imidazole antifungal agents are currently the most commonly used drugs in clinical treatment of ringworm with extensive clinical applications.
In vitro and in vivo studies have shown that luliconazole has broad-spectrum antifungal activity, with its minimum inhibitory concentration (MIC) being 0.12 to 2 mg/mL to Trichophyton (Trichophyton rubrum, Trichophyton mentagrophytes and tonsurans). Its anti-fungal effect is stronger than terbinafine, ketoconazole, miconazole, bifonazole and other commonly used drugs. Trichophyton rubrum is most sensitive to luliconazole. The MIC of Luliconazole to Candida albicans is 0.031~0.130μg/mL with the inhibitory effect being higher than that of terbinafine, Liranaftate, Butenafine, amorolfine and bifonazole, but less than that of ketoconazole, clotrimazole, neticonazole and miconazole. The MIC of Luliconazole on the important pathogens of seborrheic dermatitis, limiting Malassezia is very low, being 0.004~0.016 μg/mL with its inhibitory effect being not less but even stronger than ketoconazole.
In addition, luliconazole also have antifungal activity on filamentous fungi and yeast-like fungi with its strength being comparable as lanoconazole but higher than bifonazole and terbinafine, but being almost ineffective on Zygomycetes.
In addition, luliconazole also have antifungal activity on filamentous fungi and yeast-like fungi with its strength being comparable as lanoconazole but higher than bifonazole and terbinafine, but being almost ineffective on Zygomycetes.
Company Profile Introduction
Henan CoreyChem Co., Ltd, based on the original Zhengzhou Cote Chemical Research Institute, be brave in absorbing highly educated talents & overseas returnees; actively responded to Zhengzhou City High-tech Zone Government’s Special Care Policy, reorganized and founded in National University of Science and Technology Park, which is a high-tech, stock enterprise of high-end chemical Custom synthesis;The park was created by the People's Government of Henan Province, and proved by Ministry of Education and the National Science & Technology, taking the construction mode of "many college a park, and common development", mainly depends on Zhengzhou University and Henan University’s scientific research and talent advantage to set up Universities, scientific research institute and enterprise scientific research achievements transformation platform, to make high-tech enterprises incubate, is the new high-tech talent gathering base, high and new technology industry enterprise radiation base, colleges and universities technological innovation base.
Henan Coreychem Co., Ltd, facing global High-tech pharmaceutical raw materials, high complex new type intermediates, fine chemicals custom synthesis, scale-up production and Rare chemicals trade. Corey have well-equipped machine, strong technical force and considerate marketing team service. We also have rich experience advantage in basic research, small scale process development, scale-up, industrial technology development & production and cost control.
Recommended supplier
-
VIP1年
- SETV ASRV LLP
- LULICONAZOLE 95-99 %
- Inquiry
- 2024-09-11
-
VIP1年
- Maithri Drugs Pvt Ltd
- Luliconazole 187164-19-8 98%
- Inquiry
- 2024-03-28
-
VIP1年
- Allastir Private Limited
- 187164-19-8 98%
- Inquiry
- 2024-03-20
-
VIP1年
- Sibram Pharmaceutical
- Luliconazole 99%
- Inquiry
- 2024-03-16
-
VIP1年
- Niksan Pharmaceutical
- Luliconazole 99%
- Inquiry
- 2024-03-14
-
VIP1年
- Bioaltus Laboratories Pvt Ltd
- Luliconazole 98%
- Inquiry
- 2024-03-01
-
VIP1年
- Optimus Pharma Pvt Ltd (Sekhmet Pharmaventures))
- 187164-19-8 98%
- Inquiry
- 2024-02-28
-
VIP1年
- ProVentus Life Sciences Pvt Ltd
- 187164-19-8 Luliconazole 98%
- Inquiry
- 2024-02-28
-
VIP1年
- BDR Pharmaceuticals International Pvt Ltd
- Luliconazole 187164-19-8 98%
- Inquiry
- 2024-02-24
-
VIP1年
- Humble Healthcare Limited
- Luliconazole 99%
- Inquiry
- 2024-01-30
- Since:2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
