ZM 447439
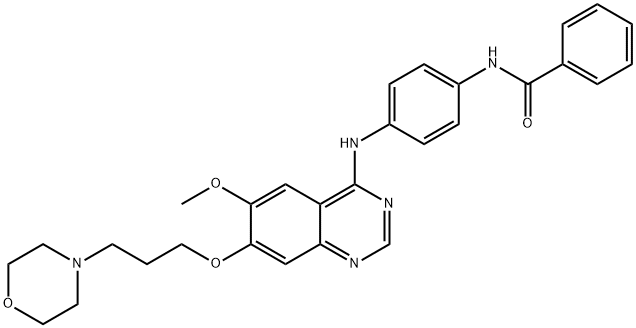
Synonym(s):4-(4-(N-Benzoylamino)anilino)-6-methoxy-7-(3-(1-morpholino)propoxy)quinazoline;Aurora Kinase Inhibitor VI, ZM447439 - CAS 331771-20-1 - Calbiochem
- CAS NO.:331771-20-1
- Empirical Formula: C29H31N5O4
- Molecular Weight: 513.59
- MDL number: MFCD08703125
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is ZM 447439?
Description
The Aurora kinases have important roles in regulating mitosis and cytokinesis, with Aurora B involved in centromere function as part of the Chromosomal Passenger Complex, with survivin, INCENP, and borealin. ZM 447439 is a selective inhibitor of Aurora B kinase (IC50 = 50 nM), less potently inhibiting Aurora C and A (IC50 = 250 and 1,000 nM, respectively). It has no effect on several other kinases, including Cdk1, Cdk2, Cdk4, Plk1, CHK1, KDR2, and FAK (IC50 > 10 μM). ZM 447439 has been used to study the role of Aurora B in molecular events associated with mitosis and cytokinesis. Moreover, ZM 447439 selectively inhibits proliferating cells rather than non-
Chemical properties
Pale Yellow Solid
The Uses of ZM 447439
It is a potent and selective inhibitor of Aurora B kinase. Cells treated with ZM-447439 progress through interphase, enter mitosis and assemble bipolar spindles but chromosome alignment, segregation a nd cytokinesis all fail. It induces apoptosos in Hep2 cancer cells and in acute myeloid leukemia cell lines but its propensity to induce polyploidy does not inevitably result in apoptosis.
The Uses of ZM 447439
It is a potent and selective inhibitor of Aurora B kinase. Cells treated with ZM-447439 progress through interphase, enter mitosis and assemble bipolar spindles but chromosome alignment, segregation and cytokinesis all fail. It induces apoptosos in Hep2 cancer cells and in acute myeloid leukemia cell lines but its propensity to induce polyploidy does not inevitably result in apoptosis.
What are the applications of Application
ZM-447439 is a selective inhibitor of ARK-1, ARK-2, and ARK-3
Definition
ChEBI: ZM447439 is a member of the class of quinazolines that is quinazoline which is substituted at positions 4, 6 and 7 by a (4-benzamidophenyl)nitrilo group, methoxy group and a 3-(morpholin-4-yl)propoxy group, respectively. It is an ATP-competitive inhibitor of Aurora A and Aurora B kinases with IC50 of 110 nM and 130 nM, respectively. It has a role as an Aurora kinase inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of benzamides, a member of quinazolines, an aromatic ether, a member of morpholines, a polyether, a secondary amino compound and a tertiary amino compound.
Storage
room temperature (desiccate)
References
1) Ditchfield et al (2003) Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores; J.Cell Biol. 161 267 2) Girdler et al. (2006) Validating Aurora B as an anti-cancer drug target; J. Cell Sci. 119 3664
Properties of ZM 447439
| Melting point: | 117-120°C |
| Boiling point: | 639.7±55.0 °C(Predicted) |
| Density | 1.282±0.06 g/cm3(Predicted) |
| storage temp. | Desiccate at RT |
| solubility | Soluble in DMSO (up to 50 mg/ml) or in Ethanol (up to 25 mg/ml). |
| form | White solid |
| pka | 13.01±0.70(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase? as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
Safety information for ZM 447439
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for ZM 447439
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 ZM-447439 99% (HPLC) CAS 331771-20-1View Details
ZM-447439 99% (HPLC) CAS 331771-20-1View Details
331771-20-1 -
 Aurora Kinase Inhibitor VI, ZM447439 CAS 331771-20-1View Details
Aurora Kinase Inhibitor VI, ZM447439 CAS 331771-20-1View Details
331771-20-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
