ZILPATEROL
- CAS NO.:117827-79-9
- Empirical Formula: C14H19N3O2
- Molecular Weight: 261.32
- MDL number: MFCD00879112
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 15:35:11
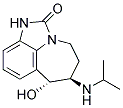
What is ZILPATEROL?
Originator
Zilpaterol hydrochloride,LONZA LTD.
The Uses of ZILPATEROL
Zilpaterol is a β-Adrenergic agonist. Growth promotant.
Definition
ChEBI: Zilpaterol is a benzazepine.
Manufacturing Process
7.6 g of sodium hydride as a 50% suspension in oil were added over 30 min
with stirring to a mixture of 29.6 g of 1,3-dihydro-1-benzyl-2H-benzimidazol-
2-one [described in Helv., Vol. 44 (1961), p. 1278] in 296 ml of
dimethylformamide and the mixture was stirred for another 30 min and was
cooled to 5°C. 33.9 g of ethyl 4-bromobutyrate were added dropwise to the
mixture over 30 min and the mixture was stirred at room temperature for 3 h
and was poured into 900 ml of iced water. The mixture was extracted with
ether and the organic phase was washed with water, dried and evaporated to
dryness. The oil residue was dissolved in 50 ml of isopropyl ether and the
solution was allowed to crystallize for 16 h and was then vacuum filtered to
obtain 22.6 g of ethyl 1,3-dihydro-2-oxo-3-benzyl-1H-benzimidazol-1-
butanoate, melting point 52°C (crystallization from cyclohexane).
A mixture of 40.6 g of the ethyl 1,3-dihydro-2-oxo-3-benzyl-1H-benzimidazol-
1-butanoate and 400 ml of 1 N methanolic sodium hydroxide was refluxed for
3 h under an inert atmosphere and was then concentrated to 0.5 its value and
was poured into 1 L of iced water. The pH was adjusted to 2 by addition of
concentrated hydrochloric acid and the mixture was vacuum filtered. The
product was washed and dried to obtain 35.2 g of 1,3-dihydro-2-oxo-3-
benzyl-1H-benzimidazol-1-butanoic acid, melting point 168°C (crystallization
from ethyl acetate).
21.5 ml of thionyl chloride were added to a suspension of 21.5 g of the product of 1,3-dihydro-2-oxo-3-benzyl-1H-benzimidazol-1-butanoic acid in
430 ml of chloroform and the mixture was refluxed for 75 min and was
evaporated to dryness under reduced pressure. The residue was dissolved in
860 ml of dichloroethane under an inert atmosphere and after cooling the
mixture to 15°C, 18.67 g of aluminum chloride were added thereto. The
mixture was stirred at 20°C for 4 h and was poured into 1 L of iced water. 43
ml of concentrated hydrochloric acid were added thereto and the mixture was
stirred for 10 min and was filtered. The decanted aqueous phase was
extracted with methylene chloride and the combined organic phases were
washed with aqueous 10% potassium carbonate to a pH of 6 and were dried
and evaporated to dryness under reduced pressure. The residue was
crystallized from ethyl acetate and dried to give 8.7 g of 5,6-dihydro-1-
benzyl-imidazo[4,5,1-j-k][1]benzazepin-2,7-[1H,4H]-dione, melting point
135°C (crystallization from isopropanol).
A mixture of 29.2 g of the 5,6-dihydro-1-benzyl-imidazo[4,5,1-j-
k][1]benzazepin-2,7-[1H,4H]-dione, 292.0 g of o-phosphoric acid and 14.1 g
of phenol were heated at 150°C under an inert atmosphere for 2 h, was
cooled to about 35°C and was poured into 1200 ml of iced water with stirring.
2 L of methylene chloride were added to the mixture which was then made
alkaline with sodium hydroxide. The mixture was filtered and the solids were
washed with methylene chloride. The combined organic phases were washed,
dried and evaporated to dryness under reduced pressure. The residue was
crystallized and was chromatographed over silica gel. Elution with a 90:2:2
ethyl acetate-methanol-triethylamine mixture yielded 9.7 g of 5,6-dihydro-
imidazo[4,5,1-j-k][1]benzazepin-2,7-[1H,4H]-dione, melting point 235°C.
42.5 ml of 1.8 N ethanolic hydrochloric acid and 10.5 ml of tert-butyl nitrite
were added at 5°C under an inert atmosphere to a suspension of 15.5 g of
the 5,6-dihydro-imidazo[4,5,1-j-k][1]benzazepin-2,7-[1H,4H]-dione in 620 ml
of tetrahydrofuran and the mixture was stirred at 5°C for 3 h and was vacuum
filtered. The product was washed with tetrahydrofuran and with a 1:1
chloroform-methanol mixture to obtain 16.5 g of 6-oxime of 4,5-
dihydroimidazo[4,5,1-j-k][1]benzazepin-2,6,7[1H]-trione, melting point
>280°C.
A suspension of 4.0 g of the 6-oxime of 4,5-dihydroimidazo[4,5,l-j-
k][1]benzazepin-2,6,7[1H]-trione 2.0 g of 10% palladium carbon and 150 ml
of methanol was stirred under hydrogen for 2,5 h and was then filtered. The
filtrate was cooled in an ice bath while slowly adding with mild stirring 0.66 g
of sodium borohydride and the mixture was stirred at 5°C for 90 min. The
mixture was evaporated to dryness under reduced pressure at 30°C and the
residue was dissolved in 15 ml of methanol. The solution was acidified to a pH
of 1-2 by addition of hydrogen chloride in ethyl acetate and the mixture was
vacuum filtered to obtain 3.6 g of (6RS, trans)-6-amino-7-hydroxy-4,5,6,7-
tetrahydro-imidazo[4,5,l-j-k][1]benzazepin-2[1H]-one hydrochloride melting
at >260°C (crystallization from methanol and then from ethanol). The base
(6RS, trans)-6-amino-7-hydroxy-4,5,6,7-tetrahydro-imidazo[4,5,l-j-
k][1]benzazepin-2[1H]-one may be produced from (6RS,trans)-6-amino-7-
hydroxy-4,5,6,7-tetrahydro-imidazo[4,5,l-j-k][1]benzazepin-2[1H]-one
hydrochloride by treatment of salt with sodium hydroxide.
3.0 g of sodium cyanoborohydride were added over 15 min at 0-5°C to a
mixture of 6.0 g of (6RS, trans)-6-amino-7-hydroxy-4,5,6,7-tetrahydro-imidazo[4,5,l-j-k][1]-benzazepin-2(1H)-one, 60 ml of methanol and 30 ml of
acetone and the mixture was stirred at room temperature for 3 h and was
evaporated to dryness under reduced pressure. The residue was added to 60
ml of water and the mixture was extracted with chloroform. The organic phase
was dried and evaporated to dryness to obtain 3.6 g of (6RS,trans)-6-
isopropylamino-7-hydroxy-4,5,6,7-tetrahydro-imidazo[4,5,l-j-k][1]-
benzazepin-2(1H)-one, melting point 166°C.
Therapeutic Function
Beta-adrenergic blocker, Cardiac stimulant
Properties of ZILPATEROL
| Melting point: | >150°C |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Chloroform (Slightly, Sonicated), DMSO (Slightly), Methanol (Slightly, Sonicated |
| form | Solid |
| color | White to Yellow |
| Stability: | Temperature Sensitive |
Safety information for ZILPATEROL
Computed Descriptors for ZILPATEROL
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 ZILPATEROL 119520-05-7 and 119520-06-8 95-99%View Details
ZILPATEROL 119520-05-7 and 119520-06-8 95-99%View Details
119520-05-7 and 119520-06-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8