Zeranol
Synonym(s):(3S,7R)-3,4,5,6,7,8,9,10,11,12-Decahydro-7,14,16-trihydroxy-3-methyl-1H-2-benzoxacyclotetradecin-1-one solution;2,4-Dihydroxy-6-(6α,10-dihydroxyundecyl)benzoic acid μ-lactone;2,4-Dihydroxy-6-(6α,10-dihydroxyundecyl)benzoic acid μ-lactone solution
- CAS NO.:26538-44-3
- Empirical Formula: C18H26O5
- Molecular Weight: 322.4
- MDL number: MFCD00864182
- EINECS: 247-769-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
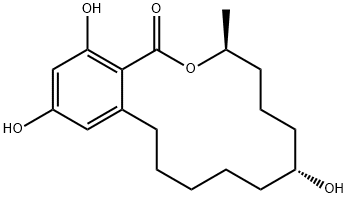
What is Zeranol?
Chemical properties
Off-White to Tan
Originator
Ralone,I.C.I. ,Italy ,1975
The Uses of Zeranol
α-Zearalanol is a minor analogue of the zearalenone family of resorcinyl macrocyclic lactones, produced by several species of Fusarium. Like the other zearalenones, α-zearalanol exhibits estrogenic activity in animals. The potency of α-zearalanol, generically known as zeranol, lead to its commercial development as a growth promotant in livestock.
The Uses of Zeranol
Animal growth promotant. Anabolic. Controlled substance
What are the applications of Application
α-Zearalanol is an estrogen receptor agonist
Definition
ChEBI: Alpha-Zearalanol is a macrolide.
Manufacturing Process
A spore sand culture containing Gibberella zeae (Gordon) NRR L-2830 was
aseptically placed in a sterile tube containing 15 ml of Czapek's-Dox solution
and a small amount of agar. This medium was then incubated for about 168
hours at approximately 25°C. At the end of the incubation period, the medium
was washed with 5 ml of sterile deionized water and transferred to a sterile
tube containing 45 ml of Czapek's-Dox solution. The contents of the tube were
then incubated for about 96 hours at about 25°C after which the material was
available for use in inoculation of a fermentation medium.
To a 2-liter flask were added 300 g of finely divided corn. The flask and its
contents were then sterilized and after sterilization 150 ml of sterile deionized
water were added. To the mixture in the flask were then added 45 ml of the
inoculum prepared by the process and the material was thoroughly mixed.
The mixed material was then incubated for about 20 days at 25°C in a dark
room in a water-saturated atmosphere. The following illustrates the recovery
of the anabolic substance from the fermentation medium.
A 300 g portion of fermented material was placed in 500 ml of deionized
water and slurried. The slurry was then heated for about 15 minutes at 75°C,
300 g of filter aid were then added and the material was filtered. The solid
filtered material containing the anabolic substance was then air dried, and 333
g of the dried cake were then extracted with 500 ml of ethanol. This
procedure was repeated three more times. The ethanol extract was then dried
under vacuum to give 6.84 g of solid material. This solid material was then
dissolved in 20 ml of chloroform and extracted with 30 ml of an aqueous
solution containing 5% by weight of sodium carbonate having an adjusted pH
of about 11.2. The extraction process was repeated seven more times. The pH
of the sodium carbonate extract was then adjusted to 6.2 with hydrochloric
acid, to yield an anabolic substance containing precipitate. The precipitate and
the aqueous sodium carbonate extract were then each in turn extracted with 75 ml of ethyl ether. This procedure was repeated three more times to yield a
light yellow ethereal solution, which was then dried to yield 116 mg of solid
anabolic substance. This material was then subjected to multiple transfer
countercurrent distribution using 100 tubes and a solvent system consisting of
two parts chloroform and two parts methanol and one part water as the upper
phase, all parts by volume. The solid material obtained from the multiple
transfer counter-current distribution was then tested for physiological activity
according to the well-known mouse-uterine test. The fermentation estrogenic
substance produced has the formula:
Tetrahydro F.E.S. was produced by dissolving 0.5 g F.E.S. in 200 ml of
ethanol. The F.E.S. was reduced by contacting the solution with hydrogen for
3 hours at 30°C and 1,000 psi using 2 g of Raney nickel as a catalyst. After
filtering and concentrating the reaction mixture, the product was washed with
2 to 3 ml of 2-nitropropane and crystallized. It was found to have a melting
point from 143°C to 160°C.
brand name
Ralabol (Pitman-Moore); Ralgro (Pitman-Moore).
Therapeutic Function
Estrogen
General Description
White fluffy powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Zeranol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Zeranol emits acrid smoke and irritating fumes.
Fire Hazard
Flash point data for Zeranol are not available; however, Zeranol is probably combustible.
Safety Profile
An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of Zeranol
| Melting point: | 182-184°C |
| Boiling point: | 381.01°C (rough estimate) |
| alpha | D +46.3° (c = 1.0 in methanol) |
| Density | 1.0919 (rough estimate) |
| refractive index | 1.6120 (estimate) |
| Flash point: | 2℃ |
| storage temp. | -20°C |
| solubility | DMF: Soluble; DMSO: Soluble; Ethanol: Soluble; Methanol: Soluble |
| form | A solid |
| pka | 8.08±0.60(Predicted) |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 26538-44-3 |
| EPA Substance Registry System | Zeranol (26538-44-3) |
Safety information for Zeranol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zeranol
Zeranol manufacturer
Varanous Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 26538-44-3 Zeranol 98%View Details
26538-44-3 Zeranol 98%View Details
26538-44-3 -
 Zeranol 98%View Details
Zeranol 98%View Details -
 α-Zearalanol CAS 26538-44-3View Details
α-Zearalanol CAS 26538-44-3View Details
26538-44-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1