Zenarestat
- CAS NO.:112733-06-9
- Empirical Formula: C17H11BrClFN2O4
- Molecular Weight: 441.639
- MDL number: MFCD00865809
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
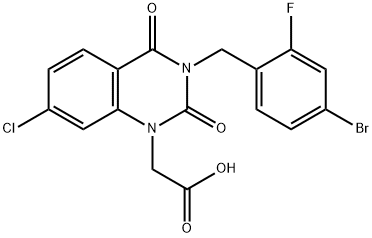
What is Zenarestat?
Originator
FK 366 ,Fujisawa Pharmaceutical
The Uses of Zenarestat
Treatment of diabetic neuropathy (aldose reductase inhibitor).
Manufacturing Process
To a solution of 4-bromo-2-fluorobenzylamine and triethylamine in chloroform
was added dropwise a solution of 4-chloro-2-nitrobenzoyl chloride in
chloroform at 0°C with stirring and the mixture was stirred at the same
temperature for 1 h. The reaction mixture was washed in turn with diluted
aqueous hydrochloric acid and water, and then dried. Evaporation of the
solvent followed by recrystallization from diethyl ether gave N-(4-bromo-2-
fluorobenzyl)-4-chloro-2-nitrobenzamide.
A mixture of N-(4-bromo-2-fluorobenzyl)-4-chloro-2-nitrobenzamide and iron
(1.45 g) in acetic acid (66 ml) was stirred at 100°C for 30 min. After cooling,
iron was filtered off. The filtrate was evaporated to give a residue, which was
made alkaline with aqueous 1 N sodium hydroxide and extracted with ethyl
acetate. The extract was washed with water and dried. Removal of the solvent
gave 2-amino-N-(4-bromo-2-fluorobenzyl)-4-chlorobenzamide.
2-Amino-N-(4-bromo-2-fluorobenzyl)-4-chlorobenzamide and N,N'-
carbonyldiimidazole were dissolved in dioxane (50 ml). The solution was
evaporated to give a residue, which was stirred at 150°C for 30 min. After
cooling, the precipitates were collected by filtration and washed with ethanol
to give 3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-2,4-
dioxoquinazoline; melting point >280°C.
To a suspension of 3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-
2,4-dioxoquinazoline in N,N-dimethylformamide was added sodium hydride
(60% in mineral oil) with stirring at 0°C and the mixture was stirred for 15
min at the same temperature. To this mixture was added ethyl bromoacetate
and the mixture was stirred for 1 h at room temperature. The reaction
mixture was poured into diluted hydrochloric acid and extracted with ethyl
acetate. The extract was washed with brine, dried and evaporated to give a
residue. Thus obtained product was purified by recrystallization from isopropyl
ether to give 2-[3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-2,4-
dioxoquinazolin-1-yl]acetic acid melting point 223°-224°C.
Therapeutic Function
Aldose reductase inhibitor
Properties of Zenarestat
| Melting point: | 223-224° |
| Boiling point: | 624.4±65.0 °C(Predicted) |
| Density | 1.737 |
| solubility | DMSO : 100 mg/mL (226.43 mM; Need ultrasonic) |
| pka | 3.76±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Zenarestat
Computed Descriptors for Zenarestat
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
![2-[(3-chlorophenyl)amino]acetic acid](https://img.chemicalbook.in/CAS/GIF/10242-05-4.gif)
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5