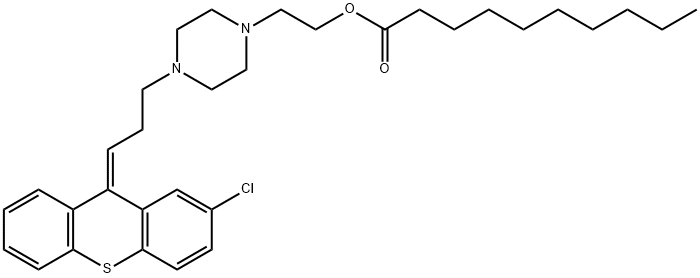
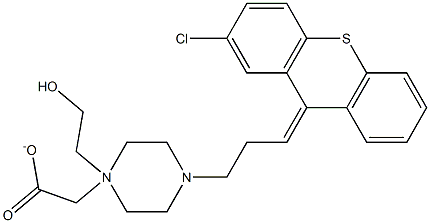
(Z)-4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]piperazine-1-ethanol dihydrochloride
- CAS NO.:58045-23-1
- Empirical Formula: C22H27Cl3N2OS
- Molecular Weight: 473.88658
- MDL number: MFCD01702794
- EINECS: 261-080-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
![(Z)-4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]piperazine-1-ethanol dihydrochloride Structural](https://img.chemicalbook.in/CAS/GIF/58045-23-1.gif)
What is (Z)-4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]piperazine-1-ethanol dihydrochloride?
Originator
Zuclopenthixol Hydrochloride,ZYF Pharm Chemical
Manufacturing Process
288.0 g of 2-chloro-9-allylthiaxanthenol-(9), melting at 77-78°C, are prepared
by adding 2-chloro-thiaxanthone to an ether solution of allyl magnesium
bromide followed by hydrolysis.
The 2-chloro-9-allylthiaxanthenol-(9) is dissolved in 2 L of anhydrous ether,
whereafter 360.0 g triethylamine are added. While stirring and cooling, 150.0
g thionyl chloride dissolved in 500 ml ether are added gradually, allowing the
temperature to rise to a maximum of -10°C. After completion of addition, the ether solution is shaken 3 times with ice water, each time with 0.3 L,
whereafter it is dried with potassium carbonate. Thereafter, the ether is
evaporated in vacuum and the 2-chloro-9-(propene-3-ylidene-1)-thiaxanthene
formed is obtained as a light yellow syrup.
2 methods of producing of 2-chloro-9-[3'-(N'-2-hydroxyethylpiperazino-
N)propylidene]thiaxanthene from 2-chloro-9-(propene-3-ylidene-1)
thiaxanthene:
1). 27.0 g of 2-chloro-9-(propene-3-ylidene-1)thiaxanthene, are mixed with
50.0 g anhydrous piperazine and 10 ml absolute ethanol and the mixture is
heated for 12 h at 120°C under reflux. After cooling, the solidified reaction
mixture is treated with 500 ml of water and the mixture extracted with ether.
From the ether solution, the 2-chloro-9-(3'-N-piperazino)propylidene)
thiaxanthene formed is extracted with dilute hydrochloric acid and precipitated
as the base from the aqueous solution by rendering the solution alkaline. By
extraction with ether, drying of the ether solution with potassium carbonate
and evaporation of the ether, the free base 2-chloro-9-(3'-N-piperazino)
propylidene)thiaxanthene is obtained as a colorless oil in a yield of 21.0 g.
35.0 g of the base 2-chloro-9-(3'-N-piperazino)propylidene)thiaxanthene are
dissolved in 200 ml of methanol. 5.0 g ethylene oxide are added and the
mixture is left standing at room temperature for 3 h. Thereafter, the reaction
mixture is evaporated, dried and the 2-chloro-9-[3'-(N'-2-
hydroxyethylpiperazino-N)propylidene]thiaxanthene is obtained.
2). 27.0 g of 2-chloro-9-(propene-3-ylidene-1)thiaxanthene, are mixed with
50.0 g anhydrous N-2-hydroxyethylpiperazine and 10 ml absolute ethanol and
the mixture is heated for 12 h at 120°C under reflux. After cooling, the
solidified reaction mixture is treated with 500 ml of water and the mixture
extracted with chloroform. From the chloroform solution, the 2-chloro-9-[3'-
(N'-2-hydroxyethylpiperazino-N)-propylidene]thiaxanthene formed is extracted
with dilute hydrochloric acid and precipitated as the base from the aqueous
solution by rendering the solution alkaline. By extraction with chloroform,
drying of the organic solution with potassium carbonate and evaporation of
the chloroform, the base 2-chloro-9-[3'-(N'-2-hydroxyethylpiperazino-
N)propylidene]thiaxanthene is obtained as colorless syrup.
By dissolving the base in petrol and leaving the solution to stand the trans
form crystallize out as a white crystalline substance, from the mother liquor
from the trans base, the corresponding cis base can be obtained as a white
crystalline substance.
In practice it is usually used as dihydrochloride.
Therapeutic Function
Neuroleptic, Antipsychotic
Properties of (Z)-4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]piperazine-1-ethanol dihydrochloride
| Melting point: | 250-260° (dec) |
| form | Solid |
| color | Light yellow to yellow |
Safety information for (Z)-4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]piperazine-1-ethanol dihydrochloride
Computed Descriptors for (Z)-4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]piperazine-1-ethanol dihydrochloride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1