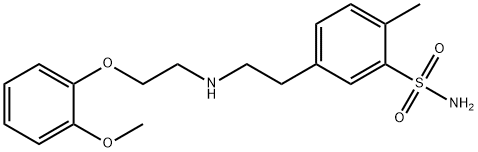
YM 09538
- CAS NO.:70958-86-0
- Empirical Formula: C18H24N2O5S.HCl
- Molecular Weight: 0
- Update Date: 2023-05-15 10:43:18
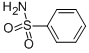
What is YM 09538?
Originator
Amosulalol hydrochloride,Yamanouchi
The Uses of YM 09538
Amosulalol Hydrochloride is used in studies to evaluate the effect of α1-blocker doxazosin on diabetic nephropathy -comparison with α,β -blockers.
Definition
ChEBI: Amosulalol hydrochloride is a sulfonamide.
Manufacturing Process
A mixture of N-benzyl-2-(2-methoxyphenoxy)ethylamine, methyl ethyl ketone,
and 5-bromoacetyl-2-methylbenzenesulfonamide was refluxed with stirring.
After cooling the reaction mixture, ethyl ketone was distilled off under reduced
pressure and the residue formed was dissolved in benzene. Then, ether was
added to the solution and after removing the hydrobromide of N-benzyl-2-(2-
methoxyphenoxy)ethylamine precipitated, the solvent was distilled off under
reduced pressure to provide a viscous oily product.
The viscous oily product was dissolved in ethanol and after adding to the
solution an excess amount of sodium borohydride, the mixture was stirred at
room temperature followed by distilling off ethanol under reduced pressure.
The residue was dissolved in ethyl acetate and the ethyl acetate layer
recovered was washed with water, dried over anhydrous sodium sulfate, and
concentrated under reduced pressure to provide a viscous oily product. The
product was subjected to a silica gel column chromatography and eluted using
benzene and then a mixture of benzene and ethyl acetate of 10:1 by volume
ratio to provide 5-{1-hydroxy-2-[N-benzyl-2-(2-
methoxyphenoxy)ethylamino]ethyl}-2-methyl-benzenesulfonamide as a
viscous oily product.
In 200 ml of methanol was dissolved 20.0 g of 5-{1-hydroxy-2-[N-benzyl-2-
(2-methoxyphenoxy)ethylamino]ethyl}-2-methyl-benzenesulfonamide. After
adding thereto 20 ml of ethanol containing about 10% hydrogen chloride and
1.0 g of 10% palladium charcoal, the mixture was shaken in hydrogen gas
stream. When the absorption of hydrogen stopped, the catalyst was filtered
away and the filtrate was distilled off under reduced pressure. The residue
was dissolved in 100 ml of ethanol while it was hot and the solution was
allowed to stand overnight in ice chamber, whereby 12.8 g of the α-type
crystals of 5-{1-hydroxy-2-[2-(2-methoxyphenoxy)ethylamino]ethyl}-2-
methylbenzenesulfonamide were obtained as the colorless crystals, melting
point 169°-171°C.
In practice it is usually used as monohydrochloride
Therapeutic Function
Alpha-adrenergic blocker, Beta-adrenergic blocker, Antihypertensive
Properties of YM 09538
| Melting point: | 158-160℃ |
Safety information for YM 09538
Computed Descriptors for YM 09538
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran
You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0