YKP-3089
- CAS NO.:913088-80-9
- Empirical Formula: C10H10ClN5O2
- Molecular Weight: 267.67
- MDL number: MFCD30533421
- Update Date: 2023-05-04 15:15:31
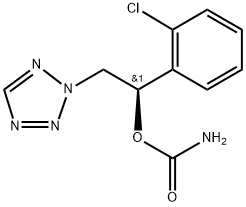
What is YKP-3089?
Absorption
Cenobamate is 88% orally bioavailable with a Tmax of 1-4 hours. A high fat meal does not significantly impact the pharmacokinetics of cenobamate.
Toxicity
There is limited information regarding the signs, symptoms, and treatment of a cenobamate overdose. Symptomatic and supportive treatment is recommended and there is limited data on the utility of dialysis to remove cenobamate from blood.
The Uses of YKP-3089
Cenobamate can be used to treat focal-onset seizures in adults.
Indications
Cenobamate is indicated for the treatment of partial onset seizures in adults.
Background
Cenobamate, or YKP-3089, is an antiepileptic drug developed by SK Pharmaceuticals and used to treat partial onset seizures. The exact mechanism of action has not been described in the literature, though it positively modulates GABAA and inhibits voltage gated sodium channels.
Cenobamate was granted FDA approval on 21 November 2019.
Pharmacokinetics
The mechanism of cenobamate is unknown, however it modulates GABAA and inhibit voltage gated sodium channels. Cenobamate is given once daily and so it has a long duration of action. The therapeutic window is wide as doses of 750mg can be well tolerated. Patients should be counselled regarding the risk of DRESS syndrome, QT interval shortening, suicidal behavior, and neurological adverse effects.
Metabolism
Data regarding the metabolism of cenobamate is lacking, however it is mostly glucuronidated by UGT2B7 and UGT2B4 or oxidized by a number of cytochromes.
Properties of YKP-3089
| Boiling point: | 520.8±60.0 °C(Predicted) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| pka | 12.76±0.50(Predicted) |
Safety information for YKP-3089
Computed Descriptors for YKP-3089
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![BenzaMide, N-[4-chloro-3-(trifluoroMethyl)phenyl]-2-[2-(diMethylaMino)ethoxy]-6-ethoxy-](https://img.chemicalbook.in/CAS/20150408/GIF/1311423-89-8.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8