Vinflunine
- CAS NO.:162652-95-1
- Empirical Formula: C45H54F2N4O8
- Molecular Weight: 816.94
- MDL number: MFCD00938122
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
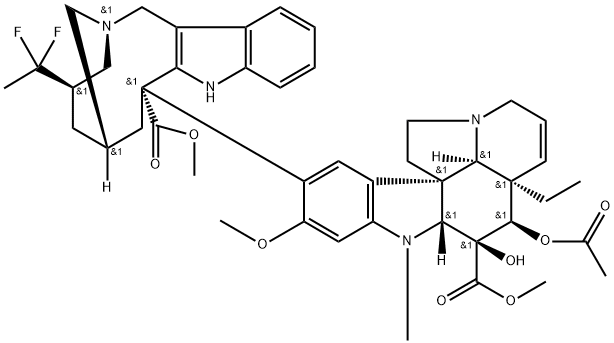
What is Vinflunine?
Absorption
Vinflunine displays a linear pharmacokinetic profile in the range of administered doses (from 30 mg/m^2 to 400 mg/m^2) in cancer patients .
Toxicity
Overdose of vinflunine is associated with bone marrow suppression with a risk of severe infection. There is no known antidote for vinflunine overdose. In case of overdose, the vital functions of the patient should be closely monitored and other appropriate measures, such as blood transfusions and administration of antibiotics or growth factors, should be taken if necessary . The severity of correlates with the AUC of, or overall exposure to, vinflunine .
In the in vivo micronucleus test in rat, vinflunine was clastogenic that induced chromosome breakage. In a mouse lymphoma assay, vinflunine displayed mutagenic and clastogenic potential without any metabolic activation . In the reproduction studies, vinflunine caused embryolethal and teratogenic effects in rabbits and teratogenic effects in rats. 2 cases of malformations of the uterus and vagina following vinflunine treatment were reported during the pre- and post-natal development study in rat .
Description
Vinflunine (also referred to as PM391) is a semisynthetic analog of the natural vinca alkaloids vinblastine and vincristine that was approved by the European Medicines Agency (EMEA) in 2009 for the treatment of adult patients with advanced or metastatic transitional cell carcinoma of the urothelial tract after failure of a prior platinum-containing regimen. Vinflunine is synthesized from anhydrovinblastine in a superacid media (HF-SbF5) and in the presence of a chlorinated solvent like CHCl3 or CCl4.
Originator
Pierre Fabre (France)
Indications
For use as a monotherapy in adults with advanced or transitional cell carcinoma of the urothelial tract after failure of a prior platinum-containing therapy .
Background
Vinflunine is a third-generation member of the vinca alkaloid family with anti-tumour actions. It was first described in 1998 at the Pierre Fabre research center in France. Like other vinca agents, vinflunine is an anti-mitotic agent that induces a cell cycle arrest at the G2/M phase and promotes cell death via apoptosis . Vinflunine is a microtubule inhibitor that binds to tubulin at or near to the vinca binding sites to inhibits its polymerization into microtubules during cell proliferation . In murine tumors and human tumor xenografts, vinflunine exhibits an antitumor efficacy than Vinorelbine, Vinblastine, and Vincristine .
Having an incidence of 429,700 new cases per year worldwide, urothelial carcinoma of the bladder is one of the most common malignancies that mostly affects individuals aged 50–79 years . Some patients with advanced urothelial carcinoma experience inadequate therapeutic response from a prior platinum-containing regimen. While these patients have a median survival of approximately 4 months and a poor prognosis , there is currently no standard therapy in patients with advanced urothelial carcinoma . In 2009, vinflunine was approved by the European Medicines Agency (EMA) as a second-line therapy of metastatic and advanced urothelial cancer after failure of platinum-based treatment . Vinflunine ditartrate is an active ingredient in the EMA-authorised product Javlor for intravenous infusion. Efficacy and safety of vinflunine has not been studied in patients with performance status of 2 or less. The clinical use of vinflunine in other urologic malignancies, such as inoperable cancer of the penis, are currently have been investigated .
Definition
ChEBI: An organic heteropentacyclic compound and an organic heterotetracyclic compound that is vinorelbine in which the tetrahydropyridine moiety of the heterotetracyclic part of the molecule has been redced to the corresponding piperidine, and in which the ethy group attached to this ring has been replaced by a 1,1-difluoroethyl group.
brand name
Javlor
Pharmacokinetics
The antitumour effects of vinflunine are dependent on concentration and exposure duration of the drug . Vinflunine mediates an anti-mitotic action by inhibiting the microtubule assembly at micromolar concentrations and reducing the rate and extent of microtubule growing events . In vivo, vinflunine displays a significant antitumor activity against a broad spectrum of human xenografts in mice both in terms of survival prolongation and tumour growth inhibition . Compared with other vinca alkaloids, vinflunine is a less-potent inductor of drug resistance in vitro .
Clinical Use
Antineoplastic agent (vinca alkaloid):
Treatment of advanced or metastatic bladder cancer
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: possible increased risk of ventricular
arrhythmias with delamanid; concentration possibly
reduced by rifampicin - avoid.
Antidepressants: concentration possibly reduced by
St Johns wort - avoid.
Antifungals: concentration increased by ketoconazole
and possibly itraconazole (increased risk of
neurotoxicity) - avoid.
Antimalarials: avoid with piperaquine with
artenimol.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: concentration possibly increased by
ritonavir - avoid.
Grapefruit juice: concentration of vinflunine
increased - avoid.
Metabolism
The metabolites of influnine are mostly cytochrome P450 3A4, but 4-O-deacetylvinflunine (DVFL) may be slowly formed by multiple esterases. DVFL is the main metabolite and is the only metabolite that retains pharmacological activity .
Metabolism
Metabolised by the cytochrome CYP3A4 isoenzyme, except for 4-O-deacetylvinflunine (DVFL), the only active metabolite and main metabolite in blood which is formed by multiple esterases. Excretion occurs via the faeces (about two-thirds) and the urine (about one-third).
Properties of Vinflunine
| Density | 1.39±0.1 g/cm3(Predicted) |
| pka | 11.36±0.60(Predicted) |
| CAS DataBase Reference | 162652-95-1(CAS DataBase Reference) |
Safety information for Vinflunine
Computed Descriptors for Vinflunine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
