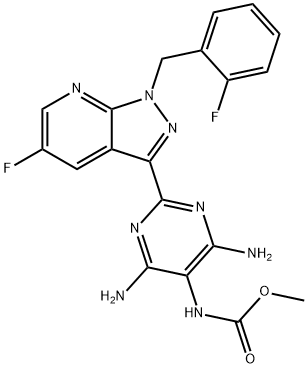
vericiguat
- CAS NO.:1350653-20-1
- Empirical Formula: C19H16F2N8O2
- Molecular Weight: 426.38
- MDL number: MFCD28502029
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
What is vericiguat?
Absorption
Following the administration of 10mg of vericiguat by mouth once daily, the average steady-state Cmax and AUC in patients with heart failure is 350 mcg/L and 6,680 mcg?h/L, respectively, with a Tmax of 1 hour. The absolute bioavailability of orally-administered vericiguat is approximately 93% when taken with food - co-administration with meals has been shown to reduce pharmacokinetic variability, increase Tmax to roughly 4 hours, and increase Cmax and AUC by 41% and 44%, respectively.
Toxicity
Data regarding overdosage with vericiguat are unavailable. Doses of up to 15mg once daily (50% greater than the recommended maintenance dose) have been studied and found to be well-tolerated. Symptoms of overdose are likely to be consistent with the adverse effect profile of vericiguat and may therefore involve significant hypotension for which symptomatic and supportive measures should be provided. Dialysis is unlikely to be of benefit in vericiguat overdose given its high degree of protein binding.
Description
Vericiguat is a novel oral soluble guanylate cyclase (sGC) stimulator.
- Soluble guanylate cyclase (sGC) is an enzyme that is activated by nitric oxide (NO). The activation initiates a signaling cascade which converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). cGMP levels increase & protein kinase G (PKG) is activated, resulting in a decrease of intracellular free Ca++ -> vascular smooth muscle cell relaxation.
- However, individuals with HF have reduced NO levels. Vericiguat, as a sGC stimulator, increases the enzymatic activity of sGC to generate cGMP independently of NO and enhances sGC sensitivity to endogenous NO.
Vericiguat is the 2nd drug in this class and follows riociguat ADEMPAS, which has been approved for treating pulmonary arterial hypertension.
SOCRATES-REDUCED was a phase II dose-finding trial of vericiguat in HF-rEF. The primary endpoint, change in NTproBNP over 12 weeks, was not statistically significant compared to placebo. A secondary exploratory analysis suggested a dose-response relationship in which higher doses of vericiguat were associated with greater reductions in NTproBNP levels.
The Uses of vericiguat
Vericiguat is a novel oral soluble guanylate cyclase (sGC) stimulator that enhances the cyclic guanosine monophosphate (GMP) production pathway, by directly stimulating soluble guanylate cyclase activity, as well as sensitizing soluble guanylate cyclase to endogenous NO. It was initially developed for potential to reduce mortality and morbidity associated with chronic heart failure with reduced ejection fraction.
The Uses of vericiguat
Vericiguat is used on targeting cyclic guanosine monophosphate in the treatment of heart failure.
Background
Vericiguat is a direct stimulator of soluble guanylate cyclase (sGC) used in the management of systolic heart failure to reduce mortality and hospitalizations. A key component of the NO-sGC-cGMP signaling pathway that helps to regulate the cardiovascular system, sGC enzymes are intracellular enzymes found in vascular smooth muscle cells (amongst other cell types) that catalyze the synthesis of cyclic guanosine monophosphate (cGMP) in response to activation by nitric oxide (NO). Cyclic GMP acts as a second messenger, activating a number of downstream signaling cascades that elicit a broad variety of effects, and these diverse cellular effects have implicated deficiencies in its production (primarily due to insufficient NO bioavailability) in the pathogenesis of various cardiovascular diseases. As a direct stimulator of sGC, vericiguat mitigates the need for a functional NO-sGC-cGMP axis and thereby helps to prevent the myocardial and vascular dysfunction associated with decreased sGC activity in heart failure.
Vericiguat was approved by the FDA in January 2021 - developed by Merck under the brand name Verquvo - for use in certain patients with systolic heart failure. Although not the first sGC stimulator to be granted FDA approval (riociguat was approved in 2013 for use in pulmonary hypertension), vericiguat is unique amongst its peers in that modifications to its structure have dramatically decreased its susceptibility to oxidative metabolism, resulting in a relatively long half-life and allowing for once-daily dosing.
Indications
Vericiguat is indicated in adults with symptomatic, chronic heart failure and an ejection fraction of <45% to reduce the risk of cardiovascular death and heart failure-related hospitalization following a hospitalization for heart failure or need for outpatient intravenous diuretics.
Definition
ChEBI: Vericiguat is a pyrazolopyridine that is 5-fluoro-1H-pyrazolo[3,4-b]pyridine in which the amino hydrogen at position 1 has been substituted by a 2-fluorobenzyl group and the hydrogen at position 3 has been substituted by a 4,6-diamino-5-[(methoxycarbonyl)amino]pyrimidin-2-yl group. It is a soluble guanylate cyclase stimulator which is used for treatment of chronic heart failure. It has a role as a soluble guanylate cyclase activator, a vasodilator agent and an antihypertensive agent. It is an aminopyrimidine, a pyrazolopyridine, a carbamate ester and an organofluorine compound.
Pharmacokinetics
By directly stimulating the increased production of intracellular cyclic guanosine monophosphate (cGMP), vericiguat causes the relaxation of vascular smooth muscle and vasodilation. Vericiguat has a relatively long half-life (~30h) that allows for once-daily dosing.
Animal reproduction studies have demonstrated the potential for embryo-fetal toxicity when vericiguat is administered to pregnant females - defects in major vessel and heart formation, as well as spontaneous abortions/resorptions, were observed when vericiguat was administered to pregnant rabbits during organogenesis. The possibility of pregnancy should be excluded prior to beginning therapy with vericiguat, and adequate contraception should be used throughout therapy and for one month following cessation of treatment.
Clinical Use
Vericiguat was advanced to clinical evaluation, as an oral therapy for chronic heart failure, either with reduced ejection fraction (HFrEF), or preserved EF (HFpEF). In mid-June 2020 Merck announced that the FDA had granted priority review status to vericiguat and based on this. The FDA approved vericiguat in January 2021, to reduce the risk of cardiovascular death, heart failure re-hospitalisation, or the requirement for outpatient intravenous diuretics, in patients with symptomatic chronic heart failure and ejection fraction less than 45%. This approval was based on efficacy data arising from the Phase 3 trial NCT02861534 (a.k.a. the VICTORIA trial).
Metabolism
Vericiguat is primarily metabolized via phase II conjugation reactions, with CYP-mediated oxidative metabolism comprising a small (<5%) portion of its overall biotransformation. The major inactive metabolite, vericiguat N-glucuronide (M1), is formed by UGT1A9 and, to a lesser extent, UGT1A1. Other identified metabolites include a denbenzylated compound and an M15 metabolite thought to be the result of oxidative metabolism, although these metabolites are poorly characterized.
Properties of vericiguat
| Boiling point: | 535.9±50.0 °C(Predicted) |
| Density | 1.63±0.1 g/cm3(Predicted) |
| solubility | DMSO:60.0(Max Conc. mg/mL);140.7(Max Conc. mM) |
| form | A solid |
| pka | 10.61±0.70(Predicted) |
| color | Light yellow to brown |
Safety information for vericiguat
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H311:Acute toxicity,dermal H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P330:Rinse mouth. P361:Remove/Take off immediately all contaminated clothing. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for vericiguat
vericiguat manufacturer
LUPA PHARMACEUTICALS
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateYou may like
-
 1350653-20-1 98%View Details
1350653-20-1 98%View Details
1350653-20-1 -
 1350653-20-1 Vericiguat 98+View Details
1350653-20-1 Vericiguat 98+View Details
1350653-20-1 -
![Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate 95% CAS 1350653-20-1](https://img.chemicalbook.in//Content/image/CP5.jpg) Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate 95% CAS 1350653-20-1View Details
Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate 95% CAS 1350653-20-1View Details
1350653-20-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
