Verapamil
- CAS NO.:52-53-9
- Empirical Formula: C27H38N2O4
- Molecular Weight: 454.61
- MDL number: MFCD00056240
- EINECS: 200-145-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18

What is Verapamil?
Absorption
More than 90% of orally administered verapamil is absorbed - despite this, bioavailability ranges only from 20% to 30% due to rapid biotransformation following first-pass metabolism in the portal circulation. Absorption kinetic parameters are largely dependent on the specific formulation of verapamil involved. Immediate-release verapamil reaches peak plasma concentrations (i.e. Tmax) between 1-2 hours following administration, whereas sustained-release formulations tend to have a Tmax between 6 - 11 hours.
AUC and Cmax values are similarly dependent upon formulation. Chronic administration of immediate-release verapamil every 6 hours resulted in plasma concentrations between 125 and 400 ng/mL. Steady-state AUC0-24h and Cmax values for a sustained-release formulation were 1037 ng?h/ml and 77.8 ng/mL for the R-isomer and 195 ng?h/ml and 16.8 ng/mL for the S-isomer, respectively.
Interestingly, the absorption kinetics of verapamil are highly stereospecific - following oral administration of immediate-release verapamil every 8 hours, the relative systemic availability of the S-enantiomer compared to the R-enantiomer was 13% after a single dose and 18% at steady-state.
Toxicity
Verapamil's reported oral TDLo is 14.4 mg/kg in women and 3.429 mg/kg in men. The oral LD50 is 150 mg/kg in rats and 163 mg/kg in mice.
As there is no antidote for verapamil overdosage, treatment is largely supportive. Symptoms of overdose are generally consistent with verapamil's adverse effect profile (i.e. hypotension, bradycardia, arrhythmia) but instances of non-cardiogenic pulmonary edema have been observed following ingestion of large overdoses (up to 9 grams). In acute overdosage, consider the use of gastrointestinal decontamination with cathartics and/or bowel irrigation. Patients presenting with significant myocardial depression may require intravenous calcium, atropine, vasopressors, or other inotropes. Consider the formulation responsible for the overdose prior to treatment - sustained-release formulations may result in delayed pharmacodynamic effects, and these patients should be monitored closely for at least 48 hours following ingestion.
Description
Verapamil, the classic calcium antagonist, has a negative inotropic, anti-ischemic, and conduction-delaying effect on the heart , . Its antiarrhythmic effect is based primarily on a prolongation of impulse conduction time in theAVnode and a reduction of frequency of impulse generation in the SA node.
Originator
Isoptin,Knoll ,W. Germany ,1963
The Uses of Verapamil
Verapamil is primarily used as an antiarrythmic for treating ventricular arrhythmias; however, currently it is being forced out gradually by adenosine.
The Uses of Verapamil
Vasodilator (coronary).
The Uses of Verapamil
Verapamil is used for preventing angina pectoris attacks, arterial hypertension, and treating and preventing supraventricular arrhythmia (paroxysmal supraventricular tachycardia, atrial fibrillation, atrial flutter, extrasystole).
Background
Verapamil is a phenylalkylamine calcium channel blocker used in the treatment of high blood pressure, heart arrhythmias, and angina, and was the first calcium channel antagonist to be introduced into therapy in the early 1960s. It is a member of the non-dihydropyridine class of calcium channel blockers, which includes drugs like diltiazem and flunarizine, but is chemically unrelated to other cardioactive medications. Verapamil is administered as a racemic mixture containing equal amounts of the S- and R-enantiomer, each of which is pharmacologically distinct - the S-enantiomer carries approximately 20-fold greater potency than the R-enantiomer, but is metabolized at a higher rate.
Indications
Verapamil is indicated in the treatment of vasopastic (i.e. Prinzmetal's) angina, unstable angina, and chronic stable angina. It is also indicated to treat hypertension, for the prophylaxis of repetitive paroxysmal supraventricular tachycardia, and in combination with digoxin to control ventricular rate in patients with atrial fibrillation or atrial flutter. Given intravenously, it is indicated for the treatment of various supraventricular tachyarrhythmias, including rapid conversion to sinus rhythm in patients with supraventricular tachycardia and for temporary control of ventricular rate in patients with atrial fibrillation or atrial flutter.
Verapamil is commonly used off-label for prophylaxis of cluster headaches.
Definition
ChEBI: A tertiary amino compound that is 3,4-dimethoxyphenylethylamine in which the hydrogens attached to the nitrogen are replaced by a methyl group and a 4-cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl group.
Manufacturing Process
177.2 g (1 mol) of veratryl cyanide are dissolved in 1 liter of toluene in a
three-neck flask. 42.9 g (1.1 mols) of pulverized sodium amide are added.
The mixture is heated to boiling under reflux for one hour while stirring and
excluding moisture. A solution of the base (N-methyl-N-homoveratryl)-γ-
aminochloropropane, freshly prepared from 339.2 g (1.1 mols) of the
hydrochloride, in 1.2 liters of toluene is added drop by drop into this boiling
mixture within two hours while stirring vigorously. Heating and stirring are
continued for four more hours. After cooling, the reaction mixture is poured
into 3 liters of ice water while stirring, The mixture is acidified with 20%
hydrochloric acid. The acidified aqueous layer is separated, neutralized by the
addition of sodium hydroxide solution, and rendered alkaline by the addition
of concentrated potassium carbonate solution. The precipitated oily base is
taken up in benzene. On evaporating the solvent, 402 g of the crude base are
obtained in the form of a reddish-brown, viscous oil.
The crude base is dissolved in a mixture of 550 ml of isopropanol and 650 ml
of ethyl acetate; Gaseous hydrogen chloride is introduced into the solution
until it is of weakly acidic reaction. On allowing the mixture to stand at 0°C,
365 g of α-[(N-methyl-N-homoveratryl)-γ-amino-propyl]-3,4-dimethoxyphenyl
acetonitrile hydrochloride precipitate as a slightly yellowish crystal powder of
the melting point 136°C to 139°C (corr.). Yield: 81% of the theoretical yield.
The pure, white hydrochloride melting at 140°C to 142°C (corr.) is obtained
on recrystallizing the crude salt twice from isopropanol with the addition of
decolorizing carbon. The salt is very soluble in water. The base prepared from
the hydrochloride in the form of an almost colorless, very viscous oil boils at
233°C to 235°C/0.01 mm Hg; nD25= 1.5532. Dioxalate, melting point: 123°C
to 125°C (corr.), on recrystallization from acetone and isopropanol.
61.9 g (0.15 mol) of α-[(N-methyl-N-homoveratryl)-γ-aminopropyl]-3,4-
dimethoxyphenyl acetonitrile are dissolved in 300 ml of toluene. The solution
is heated to boiling under reflux with 8.5 g (1.45 x 0.15 mols) of pulverized
sodium amide for one hour while stirring. Thereafter, a solution of 31.4 g (1.7
x 0.15 mols) of isopropyl bromide in 50 ml of toluene is added drop by drop
thereto within 90 minutes and the mixture is kept boiling for four more hours
while stirring. The cooled reaction mixture is allowed to run into 1.5 liters of
ice water and the mixture is acidified with 20% hydrochloric acid. The aqueous layer is separated and is rendered alkaline by the addition of a
solution of potassium carbonate. The base is taken up in warm benzene. The
solvent is evaporated and the residue is distilled in a vacuum. 62.6 g of α-
isopropyl-α-[(N-methyl-N-homoveratryl)-γ-aminopropy]-3,4-dimethoxyphenyl
acetonitrile are obtained in the form of a light yellow, very viscous oil. Boiling
point: 232°C to 235°C/0.01 mm Hg; n D 25 = 1.5460. Yield: 91.8% of the
theoretical yield. Hydrochloride: melting point: 139.5°C to 140.5°C (corr.), on
recrystallization from a mixture of isopropanol and ethyl acetate.
Therapeutic Function
Coronary vasodilator, Antiarrhythmic
General Description
Verapamil, 5-[. Hemodynamically, verapamil causesa change in the preload, afterload, contractility, heart rate,and coronary blood flow. The drug reduces systemic vascularresistance and mean blood pressure, with minor effectson cardiac output.
Verapamil is a synthetic compound possessing slightstructural similarity to papaverine. It can be separated intoits optically active isomers, of which the levorotatory enantiomeris the most potent. It is absorbed rapidly after oraladministration. The drug is metabolized quickly and, as aresult, has low bioavailability. The liver is the main siteof first-pass metabolism, forming several products. Thepreferential metabolic step involves N-dealkylation, followedby O-demethylation, and subsequent conjugation ofthe product before elimination. The metabolites have no significantbiological activity. Verapamil has an eliminationhalf-life of approximately 5 hours.
Mechanism of action
Verapamil is used as an antiarrythmic drug in treating supraventricular arrythmia such as paroxysmal atrial tachycardia, and for controlling atrial fibrillation. By blocking entrance of Ca2+ in the cell, verapamil exhibits a negative inotropic effect, and therefore it cannot be combined with β-adrenoblockers or cynidine since that would lead to an increased inotropic effect.
Pharmacokinetics
Verapamil is an L-type calcium channel blocker with antiarrhythmic, antianginal, and antihypertensive activity. Immediate-release verapamil has a relatively short duration of action, requiring dosing 3 to 4 times daily, but extended-release formulations are available that allow for once-daily dosing. As verapamil is a negative inotropic medication (i.e. it decreases the strength of myocardial contraction), it should not be used in patients with severe left ventricular dysfunction or hypertrophic cardiomyopathy as the decrease in contractility caused by verapamil may increase the risk of exacerbating these pre-existing conditions.
Clinical Use
Verapamil (Isoptin, Covera), in addition to its use as an
antiarrhythmic agent, has been employed extensively in
the management of variant (Prinzmetal’s) angina and
effort-induced angina pectoris. It selectively inhibits the voltage-gated calcium
channel that is vital for action potential genesis in slowresponse
myocytes, such as those found in the sinoatrial
and A-V nodes.
Verapamil is useful for slowing the ventricular response
to atrial tachyarrhythmias, such as atrial flutter and fibrillation.
Verapamil is also effective in arrhythmias supported
by enhanced automaticity, such as ectopic atrial
tachycardia and idiopathic left ventricular tachycardia.
Side Effects
Orally administered verapamil is well tolerated by most patients. Most complaints are of constipation and gastric discomfort. Other complaints include vertigo, headache, nervousness, and pruritus.
Synthesis
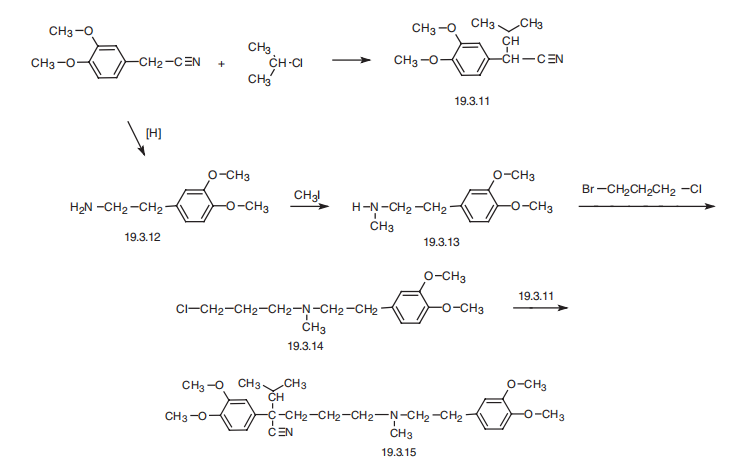
Verapamil, 5-[(3,4-dimethoxyphenethyl)methylamino]-2-(3,4-dimethoxyphenyl) isopropylvaleronitrile (19.3.15), is synthesized by a scheme using 3,4- dimethoxyphenylacetonitrile as the initial substance. The synthesis of the final product (19.3.15) is accomplished by alkylating 2-(3.4-dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) with N-[2-(3,4-dimethoxyphenyl)-ethyl]-N-3-(chloropropyl)-N-methylamine (19.3.14). The initial 2-(3.4-dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) is synthesized by alkylating 3,4-dimethoxyphenylacetonitrile with isopropyl chloride in the presence of sodium amide. The alkylating agent, N-[2-(3,4-dimethoxyphenyl)-ethyl]-N-3- (chloropropyl)-N-methylamine (19.3.14), is also synthesized from 3,4-dimethoxyphenylacetonitrile followed by reduction into 3,4-dimethoxyphenylethylamine (19.3.12), with subsequent methylation into N-methyl-N-3,4-dimethoxyphenylethylamine (19.3.13). Next, the resulting N-[2-(3,4-dimethoxyphenyl)-ethyl] -N-methylamine (19.3.12) is alkylated by 1-chloro-3-bromopropane into the desired N-[2-(3,4-dimethoxyphenyl)- ethyl]-N-3-(chloropropyl)-N-methylamine (19.3.14), which is alkylated by 2-(3.4- dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) to give the final product, verapamil(19.3.15).
Metabolism
Verapamil is extensively metabolized by the liver, with up to 80% of an administered dose subject to elimination via pre-systemic metabolism - interestingly, this first-pass metabolism appears to clear the S-enantiomer of verapamil much faster than the R-enantiomer. The remaining parent drug undergoes O-demethylation, N-dealkylation, and N-demethylation to a number of different metabolites via the cytochrome P450 enzyme system. Norverapamil, one of the major circulating metabolites, is the result of verapamil's N-demethylation via CYP2C8, CYP3A4, and CYP3A5, and carries approximately 20% of the cardiovascular activity of its parent drug. The other major pathway involved in verapamil metabolism is N-dealkylation via CYP2C8, CYP3A4, and CYP1A2 to the D-617 metabolite. Both norverapamil and D-617 are further metabolized by other CYP isoenzymes to various secondary metabolites. CYP2D6 and CYP2E1 have also been implicated in the metabolic pathway of verapamil, albeit to a minor extent. Minor pathways of verapamil metabolism involve its O-demethylation to D-703 via CYP2C8, CYP2C9, and CYP2C18, and to D-702 via CYP2C9 and CYP2C18.
Several steps in verapamil's metabolic pathway show stereoselective preference for the S-enantiomer of the given substrate, including the generation of the D-620 metabolite by CYP3A4/5 and the D-617 metabolite by CYP2C8.
Precautions
Verapamil must be used with extreme caution or not at all in patients who are receiving -adrenoceptor blocking agents. Normally, the negative chronotropic effect of verapamil will in part be overcome by an increase in reflex sympathetic tone. The latter is be prevented by simultaneous administration of a β-adrenoceptor blocking agent, which exaggerates the depressant effects of verapamil on heart rate, A-V node conduction, and myocardial contractility. The use of verapamil in children less than 1 year of age is controversial.
Properties of Verapamil
| Melting point: | 25°C |
| Boiling point: | 243-246 °C (1.3 Pa) |
| Density | 1.1267 (rough estimate) |
| refractive index | 1.5448 |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO: 100 mg/mL (219.97 mM) |
| pka | 8.6(at 25℃) |
| form | Thick Oil |
| color | Colourless |
| CAS DataBase Reference | 52-53-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Verapamil(52-53-9) |
| EPA Substance Registry System | Verapamil (52-53-9) |
Safety information for Verapamil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Verapamil
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 52-53-9 Verapamil 99%View Details
52-53-9 Verapamil 99%View Details
52-53-9 -
 VERAPAMIL 99%View Details
VERAPAMIL 99%View Details -
 Verapamil 95% CAS 52-53-9View Details
Verapamil 95% CAS 52-53-9View Details
52-53-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
