Vadadustat
- CAS NO.:1000025-07-9
- Empirical Formula: C14H11ClN2O4
- Molecular Weight: 306.7
- MDL number: MFCD30470233
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-22 10:24:25
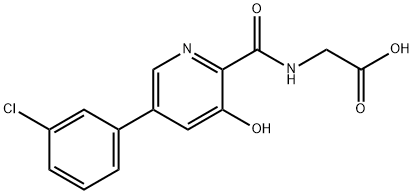
What is Vadadustat?
Absorption
Following single and repeated doses, vadadustat is rapidly absorbed. The Tmax of vadadustat ranges between 2 and 3 hr. No significant accumulation was detected in healthy subjects given repeated doses of vadadustat. Compared to fasted conditions, the administration of a 450 mg vadadustat tablet with a standard high-fat meal decreased the Cmax and AUC by 27% and 6%, respectively. Vadadustat may be taken with or without food. The mean blood-to-plasma ratio of vadadustat went from 0.50 to 0.55, suggesting that the sequestration of vadadustat into red blood cells is minimal.
Toxicity
An overdose of vadadustat may lead to extensions of the pharmacologic effects, such as increased hemoglobin levels and secondary polycythemia. In case of overdose, patients should be managed with clinically appropriate measures, such as dose reduction or treatment discontinuation. Patients should be carefully monitored and treated as clinically indicated. Approximately 16% of the vadadustat dose is removed by dialysis.
Description
Vadadustat is an inhibitor of hypoxia-inducible factor prolyl hydroxylase 2 (HIF-PH2) and HIF-PH3 (IC50s = 1.1 and 0.39 micromolar, respectively). It stabilizes HIF-1α in mouse hepatocyte nuclear extracts. Vadadustat (50 and 100 mg/kg) increases erythropoietin levels in mouse serum.
The Uses of Vadadustat
Vadadustat is a novel HIF (Hypoxia-inducible factor) stabilizer. It provides an effective anemia treatment in nondialysis-dependent chronic kidney disease.
Indications
Vadadustat is indicated for the treatment of symptomatic anemia associated with chronic kidney disease (CKD) in adults on chronic maintenance dialysis.
Background
One of the most common symptoms of advanced renal disease is anemia, caused primarily by the inability of the kidney to respond to anemic conditions with a corresponding increase in erythropoietin (EPO) production. The treatment of anemia associated with chronic kidney disease (CKD) has traditionally involved the administration of exogenous erythropoiesis-stimulating agents (ESAs), such as darbepoetin alfa, to counter the decrease in endogenous EPO production. While efficacious, the overuse of ESAs has been associated with cardiovascular complications, progression of CKD, and increases in overall mortality.
A relatively new and alternative treatment option for patients with anemia associated with CKD is the use of small molecule inhibitors of hypoxia-inducible factor prolyl-hydroxylase (HIF-PH). These agents inhibit prolyl-hydroxylase domain oxygen sensors, mimicking hypoxic conditions and activating hypoxia-inducible factors. These transcription factors serve a multitude of roles, including the stimulation of erythropoiesis.
Vadadustat is an orally administered inhibitor of HIF-PH with a safety and efficacy profile non-inferior to darbepoetin alfa for the treatment of anemia in patients with CKD undergoing dialysis. It was first approved in Japan, and in April 2023, it was approved by the EMA for the treatment of symptomatic anemia associated with CKD in adults on chronic maintenance dialysis. Vadadustat is currently awaiting a regulatory decision by the FDA. Vadadustat did not meet the prespecified noninferiority criterion for cardiovascular safety in patients with non-dialysis-dependent CKD.
Pharmacokinetics
The use of vadadustat was compared to darbepoetin alfa for the treatment of anemia in adult patients with dialysis-dependent chronic kidney disease. Vadadustat was non-inferior to darbepoetin alfa and met the primary hemoglobin level endpoint. In healthy subjects given 600 mg to 1200 mg of vadadustat, the use of this drug was not associated with clinically significant QTc prolongation. Compared to darbepoetin alfa, patients with dialysis-dependent chronic kidney disease treated with vadadustat have similar risks for death, myocardial infarction and stroke. The use of vadadustat may also lead to the development of thromboembolic events, hepatic impairment, hepatotoxicity, convulsions, as well as an increase in blood pressure.
Metabolism
Vadadustat is mainly metabolized by UDP-glucuronosyltransferase (UGT) enzymes, forming O-glucuronide conjugates via direct glucuronidation. The metabolism of vadadustat via cytochrome P450s (CYPs) is minimal. The major metabolite of vadadustat is vadadustat-O-glucuronide, which has 15% of the AUC of plasma radioactivity, and it is catalyzed by multiple UGT enzymes, including UGT1A1, UGT1A7, UGT1A8 and UGT1A9. Vadadustat acyl glucuronide is a minor metabolite with 0.047% of the total radioactivity in plasma. None of the vadadustat metabolites are active.
Properties of Vadadustat
| Melting point: | 172-174°C |
| Boiling point: | 674.6±55.0 °C(Predicted) |
| Density | 1.461±0.06 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.09±0.40(Predicted) |
| color | Pale Beige to Light Beige |
| Stability: | Hygroscopic |
Safety information for Vadadustat
Computed Descriptors for Vadadustat
Abamectin manufacturer
Ami Lifesciences Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile Diclofenac Potassium Ornidazole IP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Aceclofenac IP/BP/EP Nimesulide BP SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE Racecadotril XANTHAN GUMYou may like
-
 1000025-07-9 Vadadustat 99%View Details
1000025-07-9 Vadadustat 99%View Details
1000025-07-9 -
 ethyl 2-(3-(tert-butyl)phenoxy)-2-methylpropanoate 98%View Details
ethyl 2-(3-(tert-butyl)phenoxy)-2-methylpropanoate 98%View Details -
 89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 -
 61-68-7 98%View Details
61-68-7 98%View Details
61-68-7 -
 Diclofenac Sodium IP/BP/EP/USP 98%View Details
Diclofenac Sodium IP/BP/EP/USP 98%View Details
15307-79-6 -
 Ornidazole IP 16773-42-5 98%View Details
Ornidazole IP 16773-42-5 98%View Details
16773-42-5 -
 51803-78-2 Nimesulide BP 98%View Details
51803-78-2 Nimesulide BP 98%View Details
51803-78-2 -
 15307-81-0 98%View Details
15307-81-0 98%View Details
15307-81-0