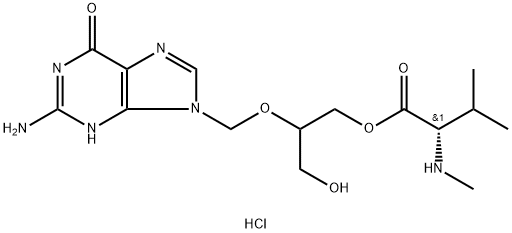
Vaborbactam
- CAS NO.:1360457-46-0
- Empirical Formula: C12H16BNO5S
- Molecular Weight: 297.14
- MDL number: MFCD28502176
- Update Date: 2024-11-19 23:02:33
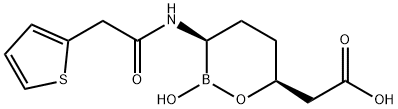
What is Vaborbactam?
Absorption
The peak plasma concentrations (Cmax) and AUC of vaborbactam increase in a dose-proportional manner. In healthy adult subjects, the Cmax following administration of multiple 2 g dose as a 3-hour infusion was 55.6 mg/L and AUC was 588 mg?h/L. In patients with the same dosing regimen, the Cmax was 71.3 mg/L and AUC was 835 mg?h/L at steady state. The exposure of vaborbactam in terms of Cmax and AUC are not expected to change with repeated dosing, and there was no evidence of accumulation of vaborbactam in plasma in a repeated dosing study.
Toxicity
In case of overdose, general supportive treatments should be initiated and hemodialysis to remove varobactam may be performed. In a pharmacokinetic study, mild lethargy observed in the highest-dose group.
The Uses of Vaborbactam
Vaborbactam is a non-β-lactam β-lactamase inhibitor. While not effective as an antibiotic by itself, it restores potency to existing antibiotics by inhibiting the beta-lactamase enzymes that would otherwise degrade them. When combined with an appropriate antibiotic it can be used for the treatment of gram-negative bacterial.
Background
Vaborbactam is a β-lactamase inhibitor based on a cyclic boronic acid pharmacophore. It has been used in trials investigating the treatment of bacterial infections in subjects with varying degrees of renal insufficiency. In August 2017, a combination antibacterial therapy under the market name Vabomere was approved by the FDA for the treatment of adult patients with complicated urinary tract infections (cUTI). Vabomere consists of vaborbactam and Meropenem for intravenous administration. Vaborbactam is added to the therapy to reduce the extent meropenem degradation by inhibiting the serine beta-lactamases expressed by the microorganism of target. The treatment aims to resolve infection-related symptoms of cUTI and achieve negative urine culture, when the infections are proven or strongly suspected to be caused by susceptible bacteria.
Indications
Indicated in combination with meropenem for the treatment of patients 18 years of age and older with complicated urinary tract infections (cUTI) including pyelonephritis caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae species complex.
Pharmacokinetics
Vaborbactam shows no antibacterial activity alone; it serves to restore the antibacterial activity of other antibacterial agents such as meropenem by attenuating their degradation by inhibiting certain serine beta-lactamases of microorganisms. Vaborbactam does not decrease the activity of meropenem against meropenem-susceptible organisms. Vaborbactam in combination with meropenem, which is a penem antibacterial drug, potentiates the bactericidal actions of meropenem against carbapenem-resistant KPC-containing Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae in a concentration-dependent manner. It restored the antimicrobial activity of meropenem in animal models of infection caused by some meropenem non-susceptible KPC-producing Enterobacteriaceae.
Metabolism
Vaborbactam does not undergo metabolism.
Properties of Vaborbactam
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Methanol (Slightly) |
| form | Solid |
| pka | 4.28±0.10(Predicted) |
| color | Pale Brown to Light Brown |
Safety information for Vaborbactam
Computed Descriptors for Vaborbactam
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 4-Fluorophenylacetic acid tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid N,N-DIMETHYLFORMAMIDE DIMETHYL ACETAL 2-Bromo-4-fluorothioanisole Para Fluoro Aniline (PFA) 2-HYDROXYPYRIDINE 4-CYANO-2-FLUORO BENZYL ALCOHOL Para Chloro Aniline (PCA) Benzamidine.HCl 5-Cyano indole 2-Methyl resorcinol Formalin; Methanal 3-Cyclopentyl-1-propanol 2-Acetylcyclohexanone 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 5-Fluoro-2-iodo-3-methylanilineRelated products of tetrahydrofuran
You may like
-
 4-methoxy-3,5-dinitropyridine 98%+View Details
4-methoxy-3,5-dinitropyridine 98%+View Details -
 2-aminopropyl benzoate hydrochloride 98%+View Details
2-aminopropyl benzoate hydrochloride 98%+View Details -
 tert-butyl 4- (ureidomethyl)benzylcarbamate 98%+View Details
tert-butyl 4- (ureidomethyl)benzylcarbamate 98%+View Details -
 1-(4-(aminomethyl)benzyl)urea hydrochloride 98%+View Details
1-(4-(aminomethyl)benzyl)urea hydrochloride 98%+View Details -
 diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate 98%+View Details
diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate 98%+View Details -
 2-(Cyanocyclohexyl)acetic acid 98%+View Details
2-(Cyanocyclohexyl)acetic acid 98%+View Details -
 (3R,4R)-1-benzyl-N,4- dimethylpiperidin-3-amine 477600-70-7 99%View Details
(3R,4R)-1-benzyl-N,4- dimethylpiperidin-3-amine 477600-70-7 99%View Details
477600-70-7 -
 Ortho Phenylene Diamine (OPDA) 95-54-5 99%View Details
Ortho Phenylene Diamine (OPDA) 95-54-5 99%View Details
95-54-5