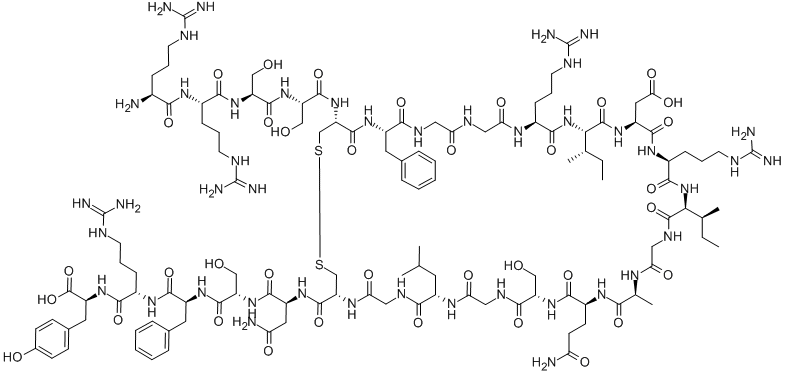
V-9-M cholecystokinin nonapeptide
- CAS NO.:99291-20-0
- Empirical Formula: C42H69N9O14S
- Molecular Weight: 956.11
- MDL number: MFCD00076493
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-16 15:32:52
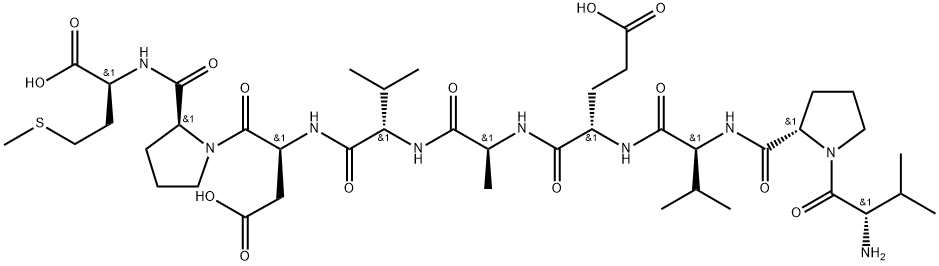
What is V-9-M cholecystokinin nonapeptide?
Description
V-9-M Cholecystokinin nonapeptide is a glucagon-like peptide receptor agonist, namely GLP-1 receptor agonist. Human GLP-1 is a peptide hormone secreted by intestinal L cells.
The Uses of V-9-M cholecystokinin nonapeptide
V-9-M cholecystokinin nonapeptide belongs to the glucagon-like peptide-1 receptor agonist (GLP-1 receptor agonist) class of hypoglycemic drugs, which increase insulin secretion in a glucose-dependent manner and inhibit glucagon secretion , and can promote gastric emptying, centrally suppress appetite, reduce food intake, and then reduce glucose absorption to achieve the effect of lowering blood sugar.
General Description
V-9-M cholecystokinin nonapeptide, also known as Cholecystokinin Precursor (24-32) (rat), is a precursor compound of cholecystokinin (CCK) that is expressed in the heart, lungs, and kidneys as well as in the gastrointestinal tract and brain. Cholecystokinin (CCK) is a brain-gut peptide that stimulates gallbladder contraction and pancreatic exocrine secretion, as well as a neurotransmitter. It can be extracted from the small intestine of dogs and cats and causes gallbladder contractions.
Mechanism of action
V-9-M cholecystokinin nonapeptide's mechanism of action is to act on islet beta cells, promote the synthesis and secretion of insulin, stimulate the proliferation and differentiation of islet beta cells, inhibit the apoptosis of islet beta cells, increase the number of islet beta cells, and protect islet function. It can also act on islet alpha cells, inhibit the release of glucagon, and reduce the release of hepatic glucose.
Solubility in organics
V-9-M cholecystokinin nonapeptide is soluble in DMSO solution, the reference solubility range is shown below:
storage
Store at low temperatures to avoid moisture:
Powder: -20°C, 3 years.
Solvents: -80°C, 2 years.
References
1. Takashima A, Itoh S. Neuropharmacological properties of V-9-M, a putative neuropeptide derived from procholecystokinin, in the rat. Can J Physiol Pharmacol. 1989 Mar;67(3):223-7. DOI: 10.1016/0301-0082(90)90035-F
2. Cholecytokinin (CCK) gene-related peptides: distribution and characterization of immunoreactive pro-CCK and an amino-terminal pro-CCK fragment in rat brain. DOI: 10.1016/0006-8993(85)90813-3
3. Cao G, Beinfeld MC. Calcium-dependent pro-cholecystokinin V-9-M immunoreactive peptide release from rat brain slices and CCK-secreting rat medullary thyroid carcinoma cells in culture. Peptides. 1992 Nov-Dec;13(6):1087-90. DOI: 10.1016/0196-9781(92)90011-Q
Properties of V-9-M cholecystokinin nonapeptide
| Boiling point: | 1350.7±65.0 °C(Predicted) |
| Density | 1.306±0.06 g/cm3(Predicted) |
| pka | 3.51±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for V-9-M cholecystokinin nonapeptide
Computed Descriptors for V-9-M cholecystokinin nonapeptide
| InChIKey | AIKMAJWJXJPJNB-HNRKYVDVNA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4