UMP
Synonym(s):U 5′-P;UMP;Uridylic acid
- CAS NO.:58-97-9
- Empirical Formula: C9H13N2O9P
- Molecular Weight: 324.18
- MDL number: MFCD00067346
- EINECS: 200-408-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
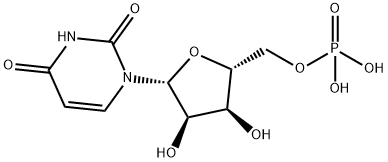
What is UMP?
Description
Uridine 5'-monophosphateis a nucleotide that is synthesized from uridine. Uridine 5'-monophosphate has been shown to have anticancer activity in vitro and in vivo, as well as antiviral activity against herpes viruses. It is also used in foods such as infant formula to as a method of increasing uridine levels in blood.
Chemical properties
(Uridine-3′-phosphate) Crystallizes in prisms from methanol. Freely soluble in water and alcohol; dextrorotatory in solution.
The Uses of UMP
Biochemical research.
The Uses of UMP
Uridine 5''-Monophosphate is a nucleotide used as monomer in RNA. It is used in foods such as infant formula to as a method of increasing uridine levels in blood.
Definition
The monophosphoric ester of uracil, i.e., the nucleotide containing uracil-d-ribose and phosphoric acid. The phosphate may be esterified to either the 2, 3, or 5 carbon of ribose, yielding uridine-2′-phosphate, uridine-3′phosphate, and uridine-5′-phosphate, respectively.
General Description
Uridine 5′-monophosphate acts as a precursor for all pyrimidine nucleotides.
Properties of UMP
| Melting point: | 202°C (rough estimate) |
| Density | 1.865±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | H2O: soluble50mg/mL, clear, colorless |
| form | solid |
| pka | pK1:6.63 (25°C) |
| color | White to Off-White |
| InChI | InChI=1S/C9H13N2O9P/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,10,12,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 |
| CAS DataBase Reference | 58-97-9 |
| EPA Substance Registry System | 5'-Uridylic acid (58-97-9) |
Safety information for UMP
Computed Descriptors for UMP
| InChIKey | DJJCXFVJDGTHFX-XVFCMESISA-N |
| SMILES | P(OC[C@H]1O[C@@H](N2C=CC(=O)NC2=O)[C@H](O)[C@@H]1O)(O)(O)=O |
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
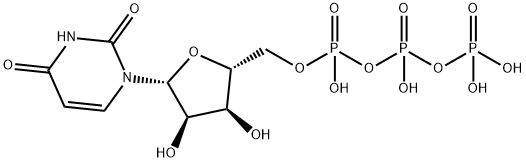
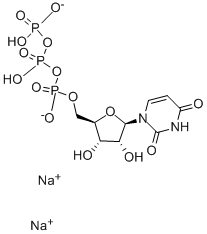
![5'-O-(4,4-Dimethoxytrityl)-2'-O-[(tert-butyl)dimethylsilyl]uridine-3'-(2-cyanoethyl-N,N-diisopropyl)phosphoramidite](https://img.chemicalbook.in/CAS/GIF/118362-03-1.gif)
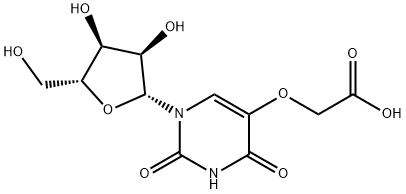
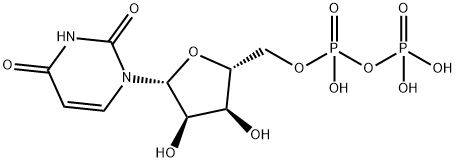
You may like
-
 Uridine 5′-monophosphate, ≥98% CAS 58-97-9View Details
Uridine 5′-monophosphate, ≥98% CAS 58-97-9View Details
58-97-9 -
 Uridine 5'-monophosphate 95% CAS 58-97-9View Details
Uridine 5'-monophosphate 95% CAS 58-97-9View Details
58-97-9 -
 Uridine 5′-monophosphate CAS 58-97-9View Details
Uridine 5′-monophosphate CAS 58-97-9View Details
58-97-9 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 Abiretorone 154229-18-2 98%View Details
Abiretorone 154229-18-2 98%View Details
154229-18-2 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
