Triphenylsilane
Synonym(s):1,1′,1′′-Silylidynetrisbenzene;NSC 12565;Triphenylhydrosilane
- CAS NO.:789-25-3
- Empirical Formula: C18H16Si
- Molecular Weight: 260.41
- MDL number: MFCD00003003
- EINECS: 212-333-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-04 09:36:04
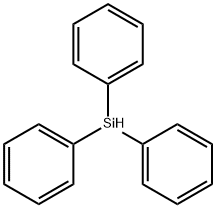
What is Triphenylsilane?
Chemical properties
Triphenylsilane is an off-white solid that is soluble in most organic solvents. It is used in certain cases for the preparation of triphenylsilyl ethers, which serve as alcohol-protecting groups. The triphenylsilyl group is considerably more stable (about 400 times) than the TMS group toward acidic hydrolysis.
The Uses of Triphenylsilane
Triphenylsilane is reducing agent for esters, xanthates, and polychloroalkanes; protecting group for alcohols. It takes part in the reactions of Deoxygenations, Hydrosilylations, Lewis Acid-assisted Hydrosilylations, Heteroatom Reductions, Multiple Bond Reductions, Alkene Hydrogenations, Silylations, etc.
The Uses of Triphenylsilane
Triphenylsilane acts as a reactant or reagent for catalytic hydrogen deuterium exchange reactions of silanes, to be oxidized by carbon nanotube-gold nanohybrids, for hydrolysis by ruthenium complexes, for hydrosilylation to produce enolsilanes, for synthesis of bromosilanes, for ozone oxidation of silyl-alkenes for synthesis of α-O-silylated acyloin derivatives. It is used to synthesize hydrido silyl and bis(silyl) bis(imidazolinylidene) nickel complexes.
What are the applications of Application
More effective radical-based reagent for reduction of organic halides than the trialkylsilanes.
Compares well with tri-n-butyltin hydride in reduction of enones to ketones.
Shows good selectivity in the reduction of cyclic hemiacetals.
Converts O-acetyl furanoses and pyranoses to deoxy sugars.
Hexaphenyldisiloxane can be conveniently made by the action of aqueous alkali on triphenylsilane, Ph3SiH.
Tristriphenylsilylbismuth, (Ph3Si)3Bi, is made similarly from triethylbismuth and triphenylsilane: Et3Bi+3Ph3SiH==Heat==(Ph3Si)3Bi+3EtH.
Preparation
Triphenylsilane can be synthesized by reducing phenylsilane (PhSiH3) with a reducing agent such as lithium aluminum hydride (LiAlH4). The overall reaction equation is as follows:
3 PhSiH3 + 2 LiAlH4 → C18H16Si + 2 LiH + 2 AlH3
This reaction is typically carried out in anhydrous conditions and under inert gas atmosphere.
Purification Methods
Purify it by recrystallisation from MeOH. [Gilman & Zuech J Am Chem Soc 81 5925 1959, Westermark Acta Chem Scand 9 947 1955, IR: Kaplan J Am Chem Soc 76 5880 1954, Beilstein 16 II 605, 16 III 1199, 16 IV 1369.]
Properties of Triphenylsilane
| Melting point: | 43-45 °C (lit.) |
| Boiling point: | 152 °C/2 mmHg (lit.) |
| refractive index | 1.6160 |
| Flash point: | 169 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | sol most organic solvents. |
| form | Powder |
| color | off-white |
| Water Solubility | REACTS |
| Sensitive | Air Sensitive |
| Hydrolytic Sensitivity | 3: reacts with aqueous base |
| BRN | 978182 |
| CAS DataBase Reference | 789-25-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, triphenyl-(789-25-3) |
| EPA Substance Registry System | Silane, triphenyl- (789-25-3) |
Safety information for Triphenylsilane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Triphenylsilane
| InChIKey | AKQNYQDSIDKVJZ-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![[(Pentafluorophenyl)thio]triphenylsilane](https://img.chemicalbook.in/CAS/GIF/22530-03-6.gif)
You may like
-
 Triphenylsilane 98% (GC) CAS 789-25-3View Details
Triphenylsilane 98% (GC) CAS 789-25-3View Details
789-25-3 -
 Triphenylsilane CAS 789-25-3View Details
Triphenylsilane CAS 789-25-3View Details
789-25-3 -
 Triphenylsilane CAS 789-25-3View Details
Triphenylsilane CAS 789-25-3View Details
789-25-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
