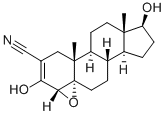
Trilostane
Synonym(s):(4α,5α,17β)-3,17-dihydroxy-4,5-epoxyandrost-2-ene-2-carbonitrile
- CAS NO.:13647-35-3
- Empirical Formula: C20H27NO3
- Molecular Weight: 329.43
- MDL number: MFCD00199295
- EINECS: 237-133-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is Trilostane?
Toxicity
Symptoms of overdose include darkening of skin, drowsiness or tiredness, loss of appetite, mental depression, skin rash, and/or vomiting.
Description
3β-
Chemical properties
Tan Crystals
Originator
Modrenal,Sterling Winthrop,UK,1980
The Uses of Trilostane
An inhibitor of steroid biosynthesis. Used as an adrenocortical suppressant. Used in the treatment of breast cancer.
The Uses of Trilostane
antifungal, inhibits mitosis in metaphase
The Uses of Trilostane
Trilostane has been used to evaluate its capability to regulate the sex-dependent differences in the lipopolysaccharide (LPS)-induced inflammatory responses of astrocytes.
Indications
Used in the treatment of Cushing's syndrome. It is normally used in short-term treatment until permanent therapy is possible.
What are the applications of Application
Trilostane is A 3β-hydroxysteroid dehydrogenase inhibitor
Background
Trilostane is an inhibitor of 3 beta-hydroxysteroid dehydrogenase used in the treatment of Cushing's syndrome. It was withdrawn from the United States market in April 1994.
Definition
ChEBI: Trilostane is an epoxy steroid that is 3,17beta-dihydroxy-5alpha-androst-2-ene-2-carbonitrile in which the oxygen of the epoxy group is joined to the 4alpha and 5 alpha positions. It has a role as an antineoplastic agent, an abortifacient and an EC 1.1.1.210 [3beta(or 20alpha)-hydroxysteroid dehydrogenase] inhibitor. It is a 3-hydroxy steroid, a 17beta-hydroxy steroid, an androstanoid, an epoxy steroid and a nitrile.
Manufacturing Process
(A)17β-acetoxy-4α,5α-epoxyandrostano[2,3-d]isoxazole, melting point 228.6°C to 229.8°C (corrected) recrystallized from a benzene-methanol
mixture, [α]D25 = +76.5°C (1% in chloroform), was prepared by treating 17β-
acetoxy-4-androsteno[2,3-d] isoxazole with maleic anhydride and hydrogen
peroxide in methylene dichloride solution.
(B)2α-cyano-4α,5α-epoxandrostan-17β-ol-3-one was prepared by treating
17β-acetoxy-4α,5α-epoxyandrostano[2,3-d] isoxazole with sodium methoxide,
and was obtained in the form of tan crystals, melting point 257.8°C to
270.0°C (decomposition) (corrected) when recrystallized from a pyridine_x0002_dioxane mixture.
brand name
Modrastane (Bioenvision).
Therapeutic Function
Corticosteroid antagonist
General Description
Trilostane inhibits the production of adrenal steroids, such as cortisol and aldosterone. It is used to treat aldosteronism.
Biochem/physiol Actions
Trilostane is an inhibitor of 3 β-hydroxysteroid dehydrogenase (3-β-HSD or delta 5-delta 4-isomerase), an essential enzyme for the biosynthesis of all classes of hormonal steroids. It has been used in the treatment of Cushing′s syndrome for stopping the production of cortisol, and is currently approved for dogs in the US, but is still a human drug in the UK and other countries. It is being investigated as a possible treatment for both breast cancer and prostate cancer to prevent the synthesis of estrogens and androgens from endogenous precursors. It has also been used to inhibit endogenous production of progesterone in research studies.
Pharmacokinetics
Trilostane blocks an enzyme involved in the production of several steroids including cortisol. Inhibiting this enzyme inhibits the production of cortisol. In Cushing's syndrome, the adrenal gland overproduces steroids. Although steroids are important for various functions of the body, too much can cause problems. Trilostane reduces the amount of steroids produced by the adrenal gland. This product was withdrawn from the U.S. market in April 1994.
Veterinary Drugs and Treatments
Trilostane may be useful for treating pituitary-dependent hyperadrenocorticism or adrenal dependent hyperadrenocorticism in dogs, feline pituitary-dependent hyperadrenocorticism, and equine hyperadrenocorticism (HAC). It may also be useful in treating Pomeranians with Alopecia X and Alaskan malamutes with adultonset alopecia.
Metabolism
Hepatic.
Properties of Trilostane
| Melting point: | 264 °C |
| Boiling point: | 467.02°C (rough estimate) |
| alpha | D25 +137.4° (c = 1 in pyridine) |
| Density | 1.1213 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥17mg/mL |
| form | powder |
| pka | 8.57±0.70(Predicted) |
| color | white to tan |
| CAS DataBase Reference | 13647-35-3(CAS DataBase Reference) |
| EPA Substance Registry System | Trilostane (13647-35-3) |
Safety information for Trilostane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Trilostane
| InChIKey | KVJXBPDAXMEYOA-CXANFOAXSA-N |
| SMILES | [C@@]123CC[C@@]4([H])[C@]5([H])CC[C@H](O)[C@@]5(C)CC[C@]4([H])[C@@]1(C)CC(C#N)=C(O)[C@@]2([H])O3 |&1:0,3,5,9,11,15,17,25,r| |
Trilostane manufacturer
Meenaakshi Molecules Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 13647-35-3 Trilostane 99%View Details
13647-35-3 Trilostane 99%View Details
13647-35-3 -
 Trilostane 99% (HPLC) CAS 13647-35-3View Details
Trilostane 99% (HPLC) CAS 13647-35-3View Details
13647-35-3 -
 Trilostane 99% (HPLC) CAS 13647-35-3View Details
Trilostane 99% (HPLC) CAS 13647-35-3View Details
13647-35-3 -
 Trilostane CAS 13647-35-3View Details
Trilostane CAS 13647-35-3View Details
13647-35-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1