THALLIUM
- CAS NO.:7440-28-0
- Empirical Formula: Tl
- Molecular Weight: 204.38
- MDL number: MFCD00134063
- EINECS: 231-138-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57

What is THALLIUM?
Description
Thallium was discovered in 1861 by Sir William Crookes (and independently by Claude-Auguste Lamy a year later) and occurs in the lithosphere at 0.7 ppm. The name thallium is in reference to the particularly bright green spectral lines seen in the spectra resultant from a flame spectroscopy test (from Greek thallos, meaning a green shoot or twig), the one used in its discovery. Thallium is a heavy metallic element that exists in the environment mainly combined with other elements (primarily oxygen, sulfur, and the halogens) in inorganic compounds.
Chemical properties
silver-grey metal, tarnishing quickly in air
Chemical properties
Thallium is a soft, bluish-white, heavy, very soft metal insoluble in water and organic solvents. It turns gray on exposure to air.
Physical properties
Thallium has much the same look (silvery) and feel as lead and is just as malleable. Unlikelead, which does not oxidize readily, thallium will oxidize in a short time, first appearing as adull gray, then turning brown, and in just a few years or less turning into blackish corrodedchunks of thallium hydroxide. This oxide coating does not protect the surface of thalliumbecause it merely flakes off exposing the next layer to oxidation.
Thallium is just to the left of lead in period 6, and both might be considered extensionsof the period 6 transition elements. Thallium’s high corrosion rate makes it unsuitable formost commercial applications. Its melting point is 304°C, its boiling point is 1,473°C, andits density is 11.85 g/cm3.
Isotopes
There are a total of 55 isotopes for thallium. All are radioactive with relativelyshort half-lives, and only two are stable. The stable ones are Tl-203, which constitutes29.524% of the element’s existence in the Earth’s crust, and Tl-205, which makes up70.476% of the element’s natural abundance found in the Earth’s crust.
Origin of Name
From the Greek word thallos, meaning “young shoot” or “green twig.” Named for the green spectral line produced by the light from the element in a spectroscope.
Occurrence
Thallium is the 59th most abundant element found in the Earth’s crust. It is widely distributedover the Earth, but in very low concentrations. It is found in the mineral/ores ofcrooksite (a copper ore; CuThSe), lorandite (TlAsS2), and hutchinsonite (lead ore, PbTl). Itis found mainly in the ores of copper, iron, sulfides, and selenium, but not in its elementalmetallic state. Significant amounts of thallium are recovered from the flue dust of industrialsmokestacks where zinc and lead ores are smelted.
History
Thallium was discovered spectroscopically in 1861 by Crookes. The element was named after the beautiful green spectral line, which identified the element. The metal was isolated both by Crookes and Lamy in 1862 about the same time. Thallium occurs in crooksite, lorandite, and hutchinsonite. It is also present in pyrites and is recovered from the roasting of this ore in connection with the production of sulfuric acid. It is also obtained from the smelting of lead and zinc ores. Extraction is somewhat complex and depends on the source of the thallium. Manganese nodules, found on the ocean floor, contain thallium. When freshly exposed to air, thallium exhibits a metallic luster, but soon develops a bluish-gray tinge, resembling lead in appearance. A heavy oxide builds up on thallium if left in air, and in the presence of water the hydroxide is formed. The metal is very soft and malleable. It can be cut with a knife. Forty-seven isotopes of thallium, with atomic masses ranging from 179 to 210 are recognized. Natural thallium is a mixture of two isotopes. The element and its compounds are toxic and should be handled carefully. Contact of the metal with skin is dangerous, and when melting the metal adequate ventilation should be provided. Thallium is suspected of carcinogenic potential for man. Thallium sulfate has been widely employed as a rodenticide and ant killer. It is odorless and tasteless, giving no warning of its presence. Its use, however, has been prohibited in the U.S. since 1975 as a household insecticide and rodenticide. The electrical conductivity of thallium sulfide changes with exposure to infrared light, and this compound is used in photocells. Thallium bromide-iodide crystals have been used as infrared optical materials. Thallium has been used, with sulfur or selenium and arsenic, to produce low melting glasses which become fluid between 125 and 150°C. These glasses have properties at room temperatures similar to ordinary glasses and are said to be durable and insoluble in water. Thallium oxide has been used to produce glasses with a high index of refraction. Thallium has been used in treating ringworm and other skin infections; however, its use has been limited because of the narrow margin between toxicity and therapeutic benefits. A mercury–thallium alloy, which forms a eutectic at 8.5% thallium, is reported to freeze at –60°C, some 20° below the freezing point of mercury. Thallium metal (99.999%) costs about $2/g.
Characteristics
Elemental thallium metal is rare in nature mainly because it oxidizes if exposed to air (oxygen)and water vapor, forming thallium oxide, a black powder. Although some compounds ofthallium are both toxic and carcinogenic, they have some uses in the field of medicine. Somecompounds have the ability to alter their electrical conductivity when exposed to infraredlight.
The Uses of THALLIUM
Thallium is used in photoelectric cells, insemiconductor studies, and in low-rangeglass thermometers. It is alloyed with manymetals. Many of its salts are used as rodent poisons. Radioactive thallium-201 is used fordiagnostic purposes in nuclear medicine inpatients with coronary artery disease.
The Uses of THALLIUM
In semi-conductor industry; alloyed with mercury for switches and closures which operate at subzero temperetures. In manufacture of highly refractive optical glass. Has been used in admixture with 97-98% of inert substances as poison for rats and other rodents.
The Uses of THALLIUM
Thallium is used as an alloy with mercury and other metals. One main use is in photoelectricapplications and for military infrared radiation transmitters.
It is also used to make artificial gemstones and special glass and to make green colors infireworks and flares. It formerly was used as a rat poison, but is no longer used for this purposebecause it is very toxic to humans.
Another main use is the radioisotope TlCl-201, with the relatively short half-life of about73 hours, in cardiac stress tests to identify potential heart abnormalities. TlCl-201 has anability to bind with the heart muscle, but only if the heart is receiving an adequate supply of blood. Restricted blood flow by blocked or narrow arteries in the heart limits the supply ofTlCl-201 absorbed. First, a small dose of TlCl-201 is injected into the patient, and the patientthen engages in a strenuous workout on a treadmill. Both before and after the test, the patientis scanned by a “gamma” detector that sends the results to a computer where the physiciancan compare the uptake of TlCl-201 before and after the treadmill stress test to determine thecondition of the patient’s heart. An area where the heart’s muscle is weak and the blood flowis limited will show up as a darkish spot on the computer screen. Since the radioisotope TlCl-201 has such a short half-life, it is soon excreted from the body. Thus, there are no long-termdetriments to the body.
What are the applications of Application
Thallium granules is a soft gray post-transition metal with atomic number 81
Definition
ChEBI: A metallic element first identified and named from the brilliant green line in its flame spectrum (from Greek thetaalphalambdalambdaomicronsigma, a green shoot).
Definition
A soft malleable grayish metallic element belonging to group 13 of the periodic table. It is found in lead and cadmium ores, and in pyrites (FeS2). Thallium is highly toxic and was used previously as a rodent and insect poison. Various compounds are now used in photocells, infrared detectors, and lowmelting glasses. Symbol: Tl; m.p. 303.5°C; b.p. 1457°C; r.d. 11.85 (20°C); p.n. 81; r.a.m. 204.3833.
Definition
thallium: Symbol Tl. A greyishmetallic element belonging togroup 13 (formerly IIIB) of the periodictable; a.n. 81; r.a.m. 204.39; r.d.11.85 (20°C); m.p. 303.5°C; b.p.1457±10°C. It occurs in zinc blendeand some iron ores and is recoveredin small quantities from lead andzinc concentrates. The naturally occurringisotopes are thallium–203and thallium–205; eleven radioisotopeshave been identified. It has fewuses – experimental alloys for specialpurposes and some minor uses inelectronics. The sulphate has beenused as a rodenticide. Thallium(I)compounds resemble those of the alkalimetals. Thallium(III) compounds are easily reduced to the thallium(I)state and are therefore strong oxidizingagents. The element was discoveredby Sir William Crookes in1861.
Production Methods
Thallium sulfide is insoluble in alkaline solution, but soluble in acid,
allowing its separation from group I elements. Thallium
chloride is only slightly soluble in cold water, which permits
its separation from chlorides of cadmium, copper, tellurium,
and zinc.
Thallium metal may be obtained from the compounds in
several ways: by electrolysis of carbonates, sulfates, or
perchlorates; by precipitation of metallic thallium with
zinc; and by reduction of thallous oxalate or chloride. A
number of industrial processes for the recovery of thallium
have been described in the literature. Several of them depend
on the extraction of thallium from flue dust by boiling it in
acidified water.
General Description
Bluish-white soft malleable metal or gray granules. Density 11.85 g / cm3. Emits toxic fumes when heated. May be packaged under water.
Air & Water Reactions
Flammable in the form of powder or dust. Insoluble in water.
Reactivity Profile
THALLIUM is a reducing agent. Reacts so vigorously with fluorine that the metal becomes incandescent [Mellor 5:421 1946-47].
Hazard
Forms toxic compounds on contact with moisture; keep from skin contact. Gastrointestinal damage and peripheral neuropathy.
Hazard
In all forms, thallium is very toxic if inhaled, when in contact with the skin, and in particular,if ingested. Mild thallium poisoning causes loss of muscle coordination and burningof the skin, followed by weakness, tremor, mental aberration, and confusion.
Thallium disease (thallotoxicosis) results from the ingestion of relatively large doses (morethan a few micrograms). The severity may vary with the age and health of the patient. Nervesbecome inflamed, hair is lost, the patient experiences stomach pain, cramps, hemorrhage,rapid heartbeat, delirium, coma, and respiratory paralysis. The disease has the potential tocause death in about one week. In the past thallium was one of the poisons of choice used bymurderers because it acts slowly and makes victims suffer. In 1987 then Iraqi dictator SaddamHussein’s agents mixed thallium powder in orange juice or yogurt and fed it to people heperceived to be his enemies. There were at least 40 thallium poisonings, mostly of Kurdishleaders. (William Langewiesche, “The Accuser,” Atlantic, March 2005, 56.)
Health Hazard
Thallium and its soluble compounds arehighly toxic in experimental animals. Theacute toxic symptoms in humans are nausea,vomiting, diarrhea, polyneuritis, convulsion,and coma. Ingestion of 0.5 g can be fatalto humans. Severe chronic toxicity can leadto kidney and liver damage, deafness, andloss of vision. Other signs of toxicity fromchronic exposure include reddening of theskin, abdominal pain, polyneuritis, loss ofhair, pain in legs, and occasionally cataracts.Ingestion of thallium salts in children hascaused neurological abnormalities, mentalretardation, and psychoses.
Hoffman (2000) reviewed thallium poisoningin women during pregnancy and cited acase that began in the first trimester of pregnancyresulting in fetal demise. John Peter andViraraghavan (2005) have reviewed toxicityof thallium and public health risk and discussedenvironmental concerns and variousremoval technologies from aquatic system.
.
Safety Profile
Human poison by unspecified route. Human systemic effects by ingestion: nerve or sheath structural changes, extra-ocular muscle changes, sweating, and other effects. Flammable in the form of dust when exposed to heat or flame. Violent reaction with F2. When heated to decomposition it emits toxic fumes of Tl. Used as a rodenticide and fungicide, and in lenses and prisms, in highdensity liquids. See also THALLIUM COMPOUNDS and POWDERED METALS.
Potential Exposure
Thallium is usually obtained as a byproduct from the flue dust generated during the roasting of pyrite ores in the smelting and refining of lead and zinc. Thallium has not been produced in the United States since 1984, but is imported for use in the manufacture of electronics, optical lenses, and imitation precious jewels. It also has use in some chemical reactions and medical procedures. Thallium and its compounds are used as a rodenticide and fungicide; in the manufacture of plates and prisms, high-density liquids; as insecticides, catalysts; in certain organic reactions, in phosphor activators; in bromoiodide crystals for lenses, plates, and prisms in infrared optical instruments; in photoelectric cells; in mineralogical analysis; alloyed with mercury in low-temperature thermometers, switches and closures; in high-density liquids; in dyes and pigments; in fire-works; and imitation precious jewelry. It forms a stainless alloy with silver and a corrosion-resistant alloy with lead. Its medicinal use for epilation has been almost discontinued. Highly persistent in the environment. Note: Thallium was used in the past as a rodenticide, it has been banned in the United States due to its toxicity from accidental exposure. In some countries, thallium(I)sulfate(2:1) is still used as a rat poison and ant bait.
Carcinogenicity
Female mice treated orally or cutaneously with high doses of thallium showed a degenerative process in the genital tract similar to that found in castrated animals or after uterine denervation. The diagnoses were papilloma, precancerous lesions, and cancer. The control mice did not develop cancer.
Environmental Fate
Metallic thallium (TI) is bluish white or gray; it is very soft
and malleable. The element can exist in the environment
mainly combined with other elements (primarily oxygen,
sulfur, and the halogens) in inorganic compounds. Thallium
exists in monovalent (thallous, thallium (I), Tl+1) and
trivalent (thallic, thallium (III), Tl+3) states. Monovalent
thallium ions also are more stable in aqueous solution, but
trivalent thallium (Tl+3) can be stabilized by complexing
agents. Monovalent thallium is similar to potassium (K+) in
ionic radius and electrical charge, which contribute to its
toxic nature.
Compounds of thallium, however, are generally soluble in
water and the element is found primarily as the monovalent
ion (Tl+). Thallium tends to adsorb to soils and sediments,
and to bioconcentrate in aquatic plants, invertebrates, and
fish. Terrestrial plants can also absorb thallium from soil. Thallium is quite stable in the environment because it is
neither transformed nor biodegraded. However, thallium may
be bioconcentrated by organisms from water. The US Environmental
Protection Agency has identified several National
Priorities List sites polluted by thallium.
Shipping
Thallium: UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN1707 Thallium compounds, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Toxicity evaluation
Several mechanisms have been postulated for the toxic action of thallium; however, the exact mechanism or mechanisms of toxicity are unknown. Thallium’s mechanism of toxicity is related to its ability to interfere with potassium ion functions because both obtain similar ionic radii. In addition, there is evidence that thallium interferes with energy production at essential steps in glycolysis, the Kreb’s cycle, and oxidative phosphorylation that adversely affects protein synthesis. Other effects include inhibition of sodium–potassium–adenosine triphosphatase and binding to sulfhydryl groups.
Incompatibilities
Varies. Cold thallium ignites on contact with fluorine. Thallium metal reacts violently with strong acids (such as hydrochloric, sulfuric, and nitric) and strong oxidizers (such as chlorine, bromine, and fluorine). Cold thallium ignites on contact with fluorine. Reacts with other halogens at room temperature.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Dilute thallium solutions may be disposed of in chemical waste landfills. When possible, thallium should be recovered and returned to the suppliers.
Properties of THALLIUM
| Melting point: | 303 °C(lit.) |
| Boiling point: | 1457 °C(lit.) |
| Density | 1.01 g/mL at 25 °C |
| solubility | insoluble in H2O; reacts with acid solutions |
| form | rod |
| color | Clear colorless |
| Specific Gravity | 11.85 |
| Resistivity | 18 μΩ-cm, 20°C |
| Water Solubility | insoluble H2O; reacts with HNO3, H2SO4 [MER06] |
| Merck | 13,9327 |
| Exposure limits | TLV-TWA 0.1 mg/m3 (thallium and its soluble
salts) (ACGIH, MSHA, and OSHA);
IDHL 10/mg/m3. |
| Stability: | Stable. |
| CAS DataBase Reference | 7440-28-0(CAS DataBase Reference) |
| EPA Substance Registry System | Thallium (7440-28-0) |
Safety information for THALLIUM
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for THALLIUM
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


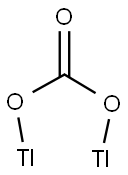

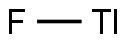
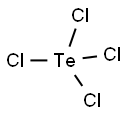
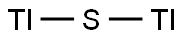

You may like
-
 Thallium CAS 7440-28-0View Details
Thallium CAS 7440-28-0View Details
7440-28-0 -
 Thallium granules CAS 7440-28-0View Details
Thallium granules CAS 7440-28-0View Details
7440-28-0 -
 Thallium granules CAS 7440-28-0View Details
Thallium granules CAS 7440-28-0View Details
7440-28-0 -
 Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
7440-28-0 -
 Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
7440-28-0 -
 Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
7440-28-0 -
 Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
Thallium rod, 12.7mm (0.5 in.) dia. CAS 7440-28-0View Details
7440-28-0 -
 Thallium Standard for ICP CASView Details
Thallium Standard for ICP CASView Details