Tetraethylammonium Chloride
Synonym(s):TEA chloride
- CAS NO.:56-34-8
- Empirical Formula: C8H20ClN
- Molecular Weight: 165.7
- MDL number: MFCD00011828
- EINECS: 200-267-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
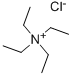
What is Tetraethylammonium Chloride?
Chemical properties
white crystalline powder
The Uses of Tetraethylammonium Chloride
Tetraethylammonium Chloride can be used as a source of tetraethylammonium ions for various pharmaceutical studies and has the ability to block K+ channels in various tissues. Tetraethylammonium Chloride can also block the transmission of nervous impulses across autonomic ganglions.
The Uses of Tetraethylammonium Chloride
Medicine (nerve-blocking agent).
The Uses of Tetraethylammonium Chloride
Tetraethylammonium chloride (TEAC) has been used:
- used for inhibition of peroxynitrite induced relaxation in rat aorta rings
- as a pharmacological blocker of K+ current and Ca2+ induced K+ current in antennal lobe neurons
- for the induction of TEAC sensitive currents in cochlear inner hair cells digested with proteolytic enzymes and analyze its properties
What are the applications of Application
Tetraethylammonium chloride is a small molecule blocker of K+ channels
Definition
ChEBI: A quarternary ammonium chloride salt in which the cation has four ethyl substituents around the central nitrogen.
General Description
Visit our Sensor Applications portal to learn more.
Biological Activity
Non-selective K + channel blocker.
Biochem/physiol Actions
Tetraethylammonium chloride (TEAC) blocks K+ channels non-specifically. In rat aorta rings, tetraethylammonium inhibits relaxation induced by peroxynitrite. It blocks nicotinic acetylcholine neurotransmission by blocking the receptor-mediated K+ currents. TEAC can increase the contractility and mobility of colon and rectum and is a therapeutic option for Hirschsprung′s disease patients. TEAC reduces the inflammatory responses, improves cardiac, vascular and hemodynamic properties during early sepsis in animals. TEAC has cytotoxic and anti-proliferative potential and induces apoptosis in glioma cells by downregulating B-cell lymphoma-2 (Bcl-2) and upregulating Bcl-2 associated X (Bax).
in vitro
tetraethylammonium (tea) is a small ion that is thought to block open k channels by binding either to an internal or to an external site. for this reason, it has been used to probe the ion conduction pathway or pore of k channel mutants and a k channel chimera. tea blocks k+ channels at two sites, which define the inner and outer mouths of the channel pores [1].
in vivo
vasorelaxant effect of taurine can be diminished by tea in rat isolated arteries [2]
Storage
Room temperature (desiccate)
Purification Methods
Crystallise the chloride from EtOH by adding diethyl ether, from warm water by adding EtOH and diethyl ether, from dimethylacetamide or from CH2Cl2 by addition of diethyl ether. Dry it over P2O5 in vacuum for several days. It also crystallises from acetone/CH2Cl2/hexane (2:2:1) [Blau & Espenson J Am Chem Soc 108 1962 1986, White & Murray J Am Chem Soc 109 2576 1987]. [Beilstein 4 IV 332.]
References
[1] taglialatela m, vandongen am, drewe ja, joho rh, brown am, kirsch ge. patterns of internal and external tetraethylammonium block in four homologous k+ channels. mol pharmacol. 1991 aug;40(2):299-307.
[2] niu lg, zhang ms, liu y, xue wx, liu db, zhang j, liang yq. vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. eur j pharmacol. 2008 feb 2;580(1-2):169-74. epub 2007 oct 25.
[3] shea pa, dunklee pe, et al. preliminary clinical investigation of tetraethylammonium chloride with particular reference to disorders of the circulatory system. calif med. 1948 sep;69(3):193-6.
Properties of Tetraethylammonium Chloride
| Melting point: | 39°C |
| Boiling point: | 273.32°C (rough estimate) |
| Density | 1.08 |
| refractive index | 1.5480 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | methanol: 0.1 g/mL, clear, colorless |
| form | Crystalline Powder |
| color | white to light yellow |
| PH | 4.0 to 7.0(50g/L, 25℃) |
| Water Solubility | Soluble in water, ethanol, chloroform, or acetone
|
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.03 |
| Merck | 14,9200 |
| BRN | 3563247 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Protect from moisture. |
| CAS DataBase Reference | 56-34-8(CAS DataBase Reference) |
| EPA Substance Registry System | Tetraethylammonium chloride (56-34-8) |
Safety information for Tetraethylammonium Chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Tetraethylammonium Chloride
| InChIKey | YMBCJWGVCUEGHA-UHFFFAOYSA-M |
Tetraethylammonium Chloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 56-34-8 99%View Details
56-34-8 99%View Details
56-34-8 -
 Tetraethylammonium chloride 96% CAS 56-34-8View Details
Tetraethylammonium chloride 96% CAS 56-34-8View Details
56-34-8 -
 Tetraethylammonium chloride, ≥98% CAS 56-34-8View Details
Tetraethylammonium chloride, ≥98% CAS 56-34-8View Details
56-34-8 -
 Tetraethylammonium chloride CAS 56-34-8View Details
Tetraethylammonium chloride CAS 56-34-8View Details
56-34-8 -
 Tetraethylammonium Chloride CAS 56-34-8View Details
Tetraethylammonium Chloride CAS 56-34-8View Details
56-34-8 -
 Tetra Ethyl Ammonium ChlorideView Details
Tetra Ethyl Ammonium ChlorideView Details
56-34-8 -
 TETRAETHYL AMMONIUM CHLORIDE (TEAC)View Details
TETRAETHYL AMMONIUM CHLORIDE (TEAC)View Details
56-34-8 -
 TETRAETHYLAMMONIUM CHLORIDEView Details
TETRAETHYLAMMONIUM CHLORIDEView Details
56-34-8
