Tavaborole
Synonym(s):1,3-Dihydro-5-fluoro-1-hydroxy-2,1-benzoxaborole;5-Fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole;AN2690;AN-2690
- CAS NO.:174671-46-6
- Empirical Formula: C7H6BFO2
- Molecular Weight: 151.93
- MDL number: MFCD10699483
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22

What is Tavaborole?
Absorption
7.5%. Subungual onychomycosis is difficult to treat due to the poorly perfused location of the infection in the nailbed. To be effective, a topical treatment must penetrate the nail plate and reach the site of infection at a concentration sufficient to exert anti-fungal activity. Tavaborole was shown to produce anti-fungal effects after 5 days of topical administration.
Toxicity
Tavaborole is generally well tolerated with most adverse events reported as mild and not related to treatment. Treatment related adverse events that occurred in >1 % of participants include application site exfoliation, application site erythema, and application site dermatitis, and ingrown toenail.
Description
Tavaborole (Kerydin®), discovered and developed by Anacor, was approved by the US FDA in July 2014. Tavaborole, topical solution, 5% is an oxaborole antifungal indicated for the treatment of onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes. The mechanism of action of tavaborole is inhibition of fungal protein synthesis, which inhibits an aminoacyl-transfer ribonucleic acid synthetase.
The Uses of Tavaborole
Tavaborole is a topical treatment of toenail onychomycosis.
Background
Tavaborale is a novel, boron-based topical antifungal medication for the treatment of onychomycosis, a fungal infection of the nail and nail bed due to Trichophyton rubrum or Trichophyton mentagrophytes infection. Tavaborole functions by inhibiting Leucyl-tRNA synthetase, or LeuRS, an essential fungal enzyme required for protein synthesis and for the catalysis of ATP-dependent ligation of L-leucine to tRNA(Leu).
Indications
Indicated for the treatment of onychomycosis (a fungal infection) of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes.
Definition
ChEBI: A member of the class of benzoxaboroles that is 1,3-dihydro-1-hydroxy-2,1-benzoxaborole substituted at position 5 by a fluoro group. A topical antifungal agent used for the treatment of onychomycosis (fungal infection of the toenails and fingernails).
General Description
Tavaborole is a boron-based pharmaceutical agent. It has broad-spectrum oxaborole antifungal activity. Due to its low molecular weight, it facilitates maximal nail plate penetration than its predecessors.
Biochem/physiol Actions
Tavaborole (AN2690) is a potent antifungal that targets the post-transfer editing site of leucyl-tRNA synthetase (LeuRS). Tavaborole forms a covalent adduct with the 3′ adenosine of tRNA(leu) at the editing site of fungal, but not bacterial LeuRS, locking the enzyme in an inactive conformation. Tavaborole was recently approved for the treatment of onychomycosis of the toenail in adults.
Pharmacokinetics
After a single dose, the mean (± standard deviation) peak concentration (Cmax) of tavaborole was 3.54 ± 2.26 ng/mL (n=21 with measurable concentrations, range 0.618-10.2 ng/mL, LLOQ=0.5 ng/mL), and the mean AUClast was 44.4 ± 25.5 nghr/mL (n=21). After 2 weeks of daily dosing, the mean Cmax was 5.17 ± 3.47 ng/mL (n=24, range 1.51--12.8 ng/mL), and the mean AUCτ was 75.8 ± 44.5 nghr/mL.
Synthesis
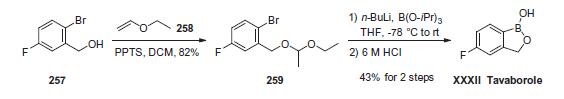
Three syntheses of tavaborole (XXXII) have been reported. The 26.9 g scale approach started with 2-bromo-5-fluorophenyl methanol (257), which was treated with ethyl vinyl ether (258) to produce o-bromobenzyl alcohol derivative 259 in 82% yield. 259 was then converted into the corresponding phenylboronic acid, followed by the one-pot deprotection and spontaneous cyclization upon treatment with 6 M hydrochloric acid, delivering tavaborole (XXXII) in 43% yield.
in vitro
an2690 showed the most active against fungi and especially against the dermatophytes t. rubrum and t. mentagrophytes, the primary fungal pathogens causing onychomycosis. in addition, an2690 was identified as having a unique profile of in vitro antidermatophyte activity, maintenance of this activity in the presence of keratin, and exceedingly good penetration of human nails [1].
Metabolism
Tavaborole undergoes extensive metabolism. Metabolite profiling revealed trace levels of a sulfated-conjugate and a benzoic acid metabolite, consistent with the known biotransformation of tavaborole.
References
[1] baker sj, zhang yk, akama t, lau a, zhou h, hernandez v, mao w, alley mr, sanders v, plattner jj. discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (an2690), for the potential treatment of onychomycosis. j med chem. 2006;49(15):4447-50.
[2] hui x, baker sj, wester rc, barbadillo s, cashmore ak, sanders v, hold km, akama t, zhang yk, plattner jj, maibach hi. in vitro penetration of a novel oxaborole antifungal (an2690) into the human nail plate. j pharm sci. 2007;96(10):2622-31.
[3] markham a. tavaborole: first global approval. drugs. 2014;74(13):1555-8.
Properties of Tavaborole
| Melting point: | 120-122℃ |
| Boiling point: | 230.8±50.0 °C(Predicted) |
| Density | 1.28±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 6.81±0.20(Predicted) |
| form | powder |
| color | white to beige |
| Stability: | Hygroscopic |
Safety information for Tavaborole
Computed Descriptors for Tavaborole
Abamectin manufacturer
Biophore India Pharmaceuticals Pvt Ltd
SETV ASRV LLP
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![7-aminobenzo[c][1,2]oxaborol-1-(3H)-ol](https://img.chemicalbook.in/CAS/20180702/GIF/947165-27-7.gif)
![5-aminobenzo[c][1,2]oxaborol-1(3H)-ol](https://img.chemicalbook.in/CAS/20180703/GIF/947165-26-6.gif)
![6-aMinobenzo[c][1,2]oxaborol-1(3H)-ol](https://img.chemicalbook.in/CAS/GIF/117098-94-9.gif)
![4-aminobenzo[c][1,2]oxaborol-1(3H)-ol](https://img.chemicalbook.in/CAS/20180808/GIF/1285533-08-5.gif)
![6-FLUOROBENZO[C][1,2]OXABOROL-1(3H)-OL](https://img.chemicalbook.in/CAS/GIF/174671-89-7.gif)
![5-(TRIFLUOROMETHYL)BENZO[C][1,2]OXABOROL-1(3H)-OL](https://img.chemicalbook.in/CAS/GIF/174671-50-2.gif)
![7-fluorobenzo[c][1,2]oxaborol-1(3H)-ol](https://img.chemicalbook.in/CAS/20180703/GIF/174671-93-3.gif)
You may like
-
 174671-46-6 98%View Details
174671-46-6 98%View Details
174671-46-6 -
 Tavaborole 174671-46-6 98%View Details
Tavaborole 174671-46-6 98%View Details
174671-46-6 -
 Tavaborole 174671-46-6 95-99 %View Details
Tavaborole 174671-46-6 95-99 %View Details
174671-46-6 -
 Tavaborole 174671-46-6 98%View Details
Tavaborole 174671-46-6 98%View Details
174671-46-6 -
 Tavaborole 98%View Details
Tavaborole 98%View Details -
 174671-46-6 98%View Details
174671-46-6 98%View Details
174671-46-6 -
 Tavaborole CAS 174671-46-6View Details
Tavaborole CAS 174671-46-6View Details
174671-46-6 -
 Tavaborole CAS 174671-46-6View Details
Tavaborole CAS 174671-46-6View Details
174671-46-6