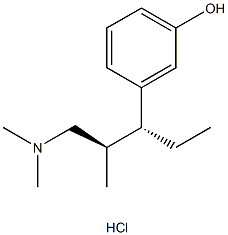
Tapentadol Hydrochloride
Synonym(s):Tapentadol hydrochloride solution
- CAS NO.:175591-09-0
- Empirical Formula: C14H24ClNO
- Molecular Weight: 257.81
- MDL number: MFCD00944992
- EINECS: 687-970-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-11-02 15:05:42
What is Tapentadol Hydrochloride?
Description
Agonism of the MOR is a common strategy for moderate to severe pain intervention. Opioid drugs, such as morphine, that modulate this receptor have demonstrated efficacy in acute situations; however, chronic conditions, particularly those of neuropathic or inflammatory etiology, suffer from inadequate pain management with this treatment. With a narrow therapeutic window, traditional MOR agonists flirt with side effects at optimal analgesia, and prolonged use increases the potential for physical dependency. Since extensive efforts to design activators of MOR have failed to dissociate the undesirable adverse effects from the analgesic properties, the focus has been on enhancing the analgesic efficacy through a dual mechanism of action. Tapentadol hydrochloride brings this concept to fruition; MOR agonism is coupled with noradrenaline reuptake inhibition in a combinatory contribution to analgesia. Compared to morphine, it is about 50-fold less potent for MOR (Ki = 100 nM for tapentadol versus 2 nM for morphine). The Ki for inhibition of noradrenaline reuptake was 500 nM while reuptake of serotonin was only weakly inhibited (Ki = 2.5μM). Despite its lower affinity for MOR, the dual mechanism has provided an efficacious profile in both acute and chronic conditions with fewer side effects.
Chemical properties
Light Brown Solid
Originator
Grunenthal GmbH (Germany)
The Uses of Tapentadol Hydrochloride
A novel, centrally acting oral analgesic with a dual mode of action that has demonstrated efficacy in preclinical and clinical models of pain relief.
brand name
Nucynta
Clinical Use
Tapentadol was approved by the FDA in November 2008 for the treatment of moderate to severe acute pain. It is a centrally acting analgesic that acts as both an agonist at the l-opiod receptor and as a norepinephrine re-uptake inhibitor, allowing it to have efficacy similar to potent narcotic analgesics but without their side effects. The drug was developed by Grunenthal and Johnson & Johnson and was marketed starting in 2009.
Side Effects
The common adverse effects of tapentadol were nausea, vomiting, somnolence, dizziness, and itching. As with all opioid medications, constipation was also an issue. Tapentadol is contraindicated in patients taking monoamine oxidase inhibitors because of the potential for adverse cardiovascular events due to additive effects on norepinephrine levels. As with other MOR agonists, it is also contraindicated in patients with paralytic ileus. In patients with a history of epilepsy or seizure, tapentadol may induce seizures. Since tapentadol causes somnolence, its combination with other sleep aids could dangerously affect breathing. Similarly, patients with existing breathing or lung problems are cautioned about using tapentadol. Furthermore, patients with past or present substance abuse or drug addiction should consult the doctor prior to use since physical dependency and addiction is a risk with tapentadol. Alcohol should be avoided due to the potential additive effect on CNS depression.
Synthesis
The synthesis of tapentadol hydrochloride begins with diethyl ketone, which is subjected to a standard Mannich condition to provide a b-dimethylamino intermediate. A Grignard reaction with 3-bromoanisole was followed by the separation of diastereomers and racemic resolution. Treatment with thionyl chloride converts the hydroxy group to its corresponding chloride, which is removed by treatment with zinc borohydride (generated from zinc chloride and sodium borohydride), with overall retention of stereochemistry. The methyl ether is cleaved in refluxing HBr to afford the tapentadol salt that is ultimately converted to the hydrochloride for formulation into 50-, 75-, and 100-mg immediate-release (IR) oral tablets.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: possible opioid withdrawal with
buprenorphine and pentazocine.
Antidepressants: possible CNS excitation or
depression with MAOIs - avoid concomitant use,
and for 2 weeks after stopping MAOI; possible
CNS excitation or depression with moclobemide;
increased sedative effects with tricyclics.
Antihistamines: increased sedative effects with
sedating antihistamines.
Antipsychotics: enhanced hypotensive and sedative
effects.
Dopaminergics: avoid with selegiline.
Nalmefene: avoid concomitant use.
Sodium oxybate: enhanced effect of sodium oxybate
- avoid concomitant use.
Metabolism
Approximately 97% of the parent compound is
metabolised by conjugation with glucuronic acid to
produce glucuronides. It is also metabolised, to a lesser
extent, via the cytochrome P450 isoenzymes CYP2C9,
CYP2C19, and CYP2D6, before further conjugation.
None of the metabolites have analgesic activity.
Approximately 70% of the dose is excreted in the urine in
the conjugated form and 3% as unchanged drug.
Properties of Tapentadol Hydrochloride
| Melting point: | 178-194°C |
| Flash point: | 9℃ |
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | DMF: 20 mg/ml; DMSO: 25 mg/ml; Ethanol: 20 mg/ml; PBS (pH 7.2): 10 mg/ml |
| form | A neat solid |
Safety information for Tapentadol Hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Tapentadol Hydrochloride
Tapentadol Hydrochloride manufacturer
BTC pharm India
Pharma Links
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidYou may like
-
 Tapentediol 98%View Details
Tapentediol 98%View Details
175591-09-0 -
 TAPENTADOL HCL 98%View Details
TAPENTADOL HCL 98%View Details -
 Tapentadol hydrochloride 98%View Details
Tapentadol hydrochloride 98%View Details -
 Tapentadol hydrochloride 175591-09-0 99%View Details
Tapentadol hydrochloride 175591-09-0 99%View Details
175591-09-0 -
 175591-09-0 Tapentadol Hydrochloride 95-99%View Details
175591-09-0 Tapentadol Hydrochloride 95-99%View Details
175591-09-0 -
 TAPENTADOL 175591-09-0 95-99 %View Details
TAPENTADOL 175591-09-0 95-99 %View Details
175591-09-0 -
 Tapentadol hydrochloride - * psy CAS 175591-09-0View Details
Tapentadol hydrochloride - * psy CAS 175591-09-0View Details
175591-09-0 -
 Tapentadol Hydrochloride 98+View Details
Tapentadol Hydrochloride 98+View Details
175591-09-0
