Mobocertinib
- CAS NO.:1847461-43-1
- Empirical Formula: C32H39N7O4
- Molecular Weight: 585.71
- MDL number: MFCD32669806
- Update Date: 2024-11-19 23:02:33
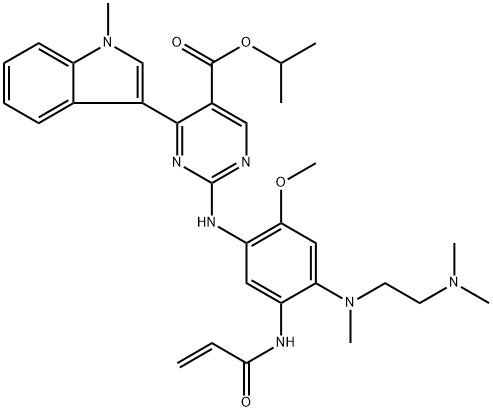
What is Mobocertinib?
Absorption
The mean absolute bioavailability of mobocertinib is 37% and the median Tmax is approximately 4 hours. Following a single oral dose of 160mg of mobocertinib to fasted patients, the mean Cmax and AUC0-inf were 45.8 ng/mL and 862 ng?h/mL, respectively.
Toxicity
No data are available regarding overdosage with mobocertinib. Symptoms of overdosage are likely to be consistent with mobocertinib's adverse effects and may therefore include significant gastrointestinal symptoms, pain, fatigue, and rash.
Description
Mobocertinib is derived from osimertinib, a second-generation TKI which exhibits only limited activity against several resistant EGFRex20ins mutants. Both inhibitors are structurally identical except that the pyrimidine ring of moboceritinib incorporates a snugly fitting isopropyl ester group that targets a previously unoccupied pocket, resulting in expanded coverage of EGFRex20ins mutations as well as improved selectivity over wildtype EGFR vs. osimertinib.
The Uses of Mobocertinib
Mobocertinib (TAK-788) is an orally active and irreversible EGFR/HER2 inhibitor. Mobocertinib effectively inhibits oncogenic variants containing mutations that activate the EGFRex20ins, with selectivity superior to that of wild-type EGFR. Mobocertinib is FDA-approved for the treatment of patients with non-small cell lung cancer (NSCLC) who have a HER2 mutation or an EGFR mutation, including an exon 20 insertion mutation. Mobocertinib is approved by the FDA for the treatment of patients with non-small cell lung cancer (NSCLC) who have HER2 mutations or EGFR mutations, including exon 20 insertion mutations.
Background
Mobocertinib is a kinase inhibitor targeted against human epidermal growth factor receptor (EGFR). It is used specifically in the treatment of non-small cell lung cancer (NSCLC) caused by exon 20 insertion mutations in the EGFR gene, which are typically associated with a poorer prognosis (as compared to "classical" EGFR mutants causing NSCLC) and are associated with resistance to standard targeted EGFR inhibitors. Mobocertinib appears to be an effective means of treating this otherwise treatment-resistant NSCLC, exerting an inhibitory effect on EGFR exon 20 insertion mutant variants at concentrations 1.5- to 10-fold lower than those required to inhibit wild-type EGFR.
Mobocertinib, under the brand name Exkivity (Takeda Pharmaceuticals Inc.), was granted accelerated approval by the FDA in September 2021 for the treatment of locally advanced or metastatic NSCLC in patients with EGFR exon 20 insertion mutations who have failed previous therapies.
Indications
Mobocertinib is indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy.
brand name
ExkivityTM
General Description
Class: receptor tyrosine kinase
Treatment: NSCLC with EGFR alterations
Oral bioavailability = 37%
Elimination half-life = 18 h
Biological Activity
In NSCLC cell lines, mobocertinib was highly active against common EGFR-activating mutations (exon 19 deletions and L858R) (IC50 = 1.3–4.0 nM) or with a gatekeeper T790M mutant (IC50 = 9.8 nM), as well as selective for wt EGFR (IC50 = 35 nM). In Ba/F3 cells, mobocertinib displayed activity against 14 EGFR mutations with IC50 values ranging from 2.7 to 22.5 nM, vs. 34.5 nM for wt EGFR. More specifically, mobocertinib inhibited all five variants of EGFRex20ins mutations with IC50 ranging from 4.3 nM to 22.5 nM.
Pharmacokinetics
Mobocertinib is an inhibitor of EGFR that preferentially targets exon 20 insertion mutant variants. It is available as an oral capsule taken with or without food once daily.
Mobocertinib can cause a concentration-dependent increase in QTc interval which may lead to life-threatening complications such as Torsades de Pointes. Patients with baseline risk factors for QTc prolongation should consider alternative medications or be monitored carefully throughout therapy. The use of concomitant QTc-prolonging medications should be avoided, as should concomitant inhibitors of CYP3A, as these may increase the concentration of mobocertinib and thus the risk of QTc-prolongation.
Metabolism
Mobocertinib is metabolized primarily by CYP3A enzymes to two active metabolites, AP32960 and AP32914, which are equipotent to mobocertinib and account for 36% and 4% of its combined molar AUC, respectively.
Metabolism
Moboceritinib undergoes CYP450-mediated metabolism to give two primary N-demethylated metabolites, AP32914 and AP32960, whose IC50 values are within 2-fold of mobocertinib for both wt and mutant EGFR. It is likely that these metabolites also contribute to the pharmacologic activity of mobocertinib. Interestingly, the typically labile isopropyl ester appears resistant to esterase-induced hydrolysis.
Properties of Mobocertinib
| storage temp. | Store at -20°C |
| solubility | DMF: 10 mg/ml,DMF:PBS (pH 7.2) (1:8): 0.11 mg/ml |
| form | A crystalline solid |
| color | Off-white to light yellow |
Safety information for Mobocertinib
Computed Descriptors for Mobocertinib
Mobocertinib manufacturer
Aspen Biopharma Labs Pvt Ltd
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![6-[(7S)-7-Hydroxy-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-7-yl]-N-methyl-2-naphthalenecarboxamide](https://img.chemicalbook.in/CAS/GIF/566939-85-3.gif)
![Benzoic acid, 4-[[(cyclopropylmethyl)[4-(2-fluorophenoxy)benzoyl]amino]methyl]-](https://img.chemicalbook.in/CAS/20200515/GIF/1664335-55-0.gif)
You may like
-
 1847461-43-1 Mobocertinib 98%View Details
1847461-43-1 Mobocertinib 98%View Details
1847461-43-1 -
 Mobocertinib 1847461-43-1 98%View Details
Mobocertinib 1847461-43-1 98%View Details
1847461-43-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1