Streptozotocin
Synonym(s):N-(Methylnitrosocarbamoyl)-α-D -glucosamine;Streptozotocin
- CAS NO.:18883-66-4
- Empirical Formula: C8H15N3O7
- Molecular Weight: 265.22
- MDL number: MFCD00006607
- EINECS: 242-646-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
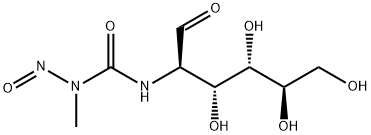
What is Streptozotocin?
Description
Streptozotocin (STZ) was originally identified in the late 1950s as an antibiotic and was discovered in a strain of the soil microbe Streptomyces achromogenes. In the mid-1960s, STZ was found to be selectively toxic to the beta cells of the pancreatic islets and thus it is used in animal model of diabetes and as a medical treatment for cancers of the beta cells. STZ’s use in cancer chemotherapy received Food and Drug Administration approval in July 1982 and the drug was subsequently marketed as Zanosar.
Chemical properties
off-white to pale yellow crystalline powder
Originator
Zanosar,Upjohn,US,1982
The Uses of Streptozotocin
Streptozotocin is used in the treatment metastatic cancer of the pancreatic islet cells. Streptozotocin has a highlrisk of toxicity and is generally limited to cancers that are inoperable.
The Uses of Streptozotocin
antineoplastic, alkylating agent
The Uses of Streptozotocin
Streptozotocin is an unusual aminoglycoside containing a nitrosoamino group produced by Streptomyces achromogenes and discovered in 1959 as an antibiotic. The nitrosoamino group enables the metabolite to act as a nitric oxide (NO) donor. NO is an important messenger molecule involved in many physiological and pathological processes in the body. Streptozotocin is also widely used to induce diabetes in rodent models by inhibition of β-cell O-GlcNAcase.
The Uses of Streptozotocin
somatostatin octapeptide analog
What are the applications of Application
Streptozotocin (U-9889) is an N-nitroso containing antibiotic and can be used as a potent DNA methylating agent.
Definition
ChEBI: An antibiotic that is produced by Streptomyces achromogenes. It is used as an antineoplastic agent and to induce diabetes in experimental animals.
Indications
Streptozocin (Zanosar), a water-soluble nitrosourea
produced by the fungus Streptomyces achromogenes,
acts through methylation of nucleic acids and proteins.
In addition, it produces rapid and severe depletion of
the pyridine nucleotides nicotinamide adenine dinucleotide
(NAD) and its reduced form (NADH) in liver
and pancreatic islets.
Streptozocin is not well absorbed from the gastrointestinal
tract and must be administered intravenously or
intraarterially. In preclinical studies, the plasma half-life
was 5 to 10 minutes.
Streptozocin produces remission in 50 to 60% of patients
with islet cell carcinomas of the pancreas. It is also
useful in malignant carcinoid tumors.
Almost all patients have nausea and vomiting. The
major toxicity is renal tubular damage, which may be severe
in 5 to 10% of patients taking streptozocin.
Treatment of metastatic insulinomas may result in the
release of insulin from the tumor and subsequent hypoglycemic
coma. Less severe toxicities include diarrhea,
anemia, and mild alterations in glucose tolerance or
liver function tests.
Manufacturing Process
On a sterile maltose-tryptone agar slant of the following composition: 1 g
maltose; 0.5 g tryptone; 0.05 g K2HPO4; 0.01 g FeSO4·7H2O; 1.5 g agar; and
sufficient distilled water to make 100 ml, Streptomyces achromogenes var.
streptozoticus was grown for 7 days at 28°C.
The culture thus produced was used as an inoculum for the following sterile
medium: 1 g glucose; 1 g beef extract; 0.5 g Bacto peptone (Difco); 0.5 g
NaCl; and sufficient distilled water to make 100 ml. The pH was adjusted to
7.0 before sterilization. The inoculated medium was incubated in shake flasks
for 3 days at 28°C on a reciprocating shaker and 75 ml of the resulting
growth was used to inoculate 12 l of sterile medium of the same formulation.
The medium was incubated in a 20 l stainless steel bottle, at 28°C for 2 days,
the contents being stirred continuously with sparged air at the rate of 6 l of
free air per minute. The resulting growth was used to inoculate 250 l of the
following sterile medium. 2 g Bacto peptone (Difco); 2.5 g blackstrap
molasses; 2 g glucose; 0.25 g NaCl; and sufficient distilled water to make 100
ml. The pH was adjusted to 7.0 before sterilization.
This medium was incubated in a 100 gallon stainless steel fermentor, at 24°C
with sparged air being introduced at the rate of 50 l/min and with agitation by
an impeller. After 66 hours of fermentation the beer was harvested. To 100
gallons of harvested beer was added 17 pounds of diatomite, and 35 pounds
of activated carbon. The mixture was stirred well and then filtered, the cake
was water-washed with 10 gallons of tap water, and then washed with 25
gallons of acetone followed by 30 gallons of 1:1 aqueous acetone. The
acetone solutions of streptozotocin were pooled and dried in vacuo to 3.88
pounds.
brand name
Zanosar (Sicor).
Therapeutic Function
Antineoplastic
General Description
Off-white powder. Melting point 115°C. Used as an anti-cancer drug. Carcinogenic.
General Description
Streptozocin is available in 1-g vials for IV administrationin the treatment of metastatic islet cell carcinoma of the pancreas,colon cancer, and Hodgkin’s disease. Activation resultsin the formation of methyl carbonium ions, which alkylateDNA, but the agent is less effective in alkylating DNAand carbamylating proteins than other nitrosoureas, which isbecause in part of an intramolecular carbamoylation. The internalcarbamate group migration occurs via the hydroxylgroups of the glucose portion of the molecule. There is a reducedeffect on RNA function compared with other nitrosoureasas well. The hydrophilic agent is rapidly clearedfrom the plasma with an elimination half-life of 35 minutes.The presence of the glucose moiety allows for utilization ofglucose transporters, which concentrate the compound inthe -cells of the pancreas. The metabolism of the agent hasnot been well characterized. Renal toxicity is dose limitingand may present initially as proteinuria and azotemia butmay progress to renal failure. Nausea and vomiting may besevere with the agent, whereas myelosuppression is generallymild. Normal glucose metabolism may be altered sothat hypoglycemia or hyperglycemia may be seen. In someanimal species, streptozocin produces diabetes but muchmilder effects are seen in man.
Air & Water Reactions
Water soluble.
Reactivity Profile
[D-GLUCOSE, 2-DEOXY-2-[[(METHYLNITROSOAMINO)-CARBONYL]AMINO] is weakly basic. Reacts exothermically with acids. Reacts with both strong oxidizing agents and strong reducing agents.
Biological Activity
Antibiotic and antitumor agent. Alkylates DNA and induces diabetes mellitus via reduction of nicotinamide adenine dinucleotide in pancreatic β -cells in vivo .
Biochem/physiol Actions
An N-nitroso-containing compound that acts as a nitric oxide donor in pancreatic islets; induces death of insulin-secreting cells, producing an animal model of diabetes. Potent DNA methylating agent that induces chromosomal breakage. Cytotoxic to neuroendocrine tumor cell lines that express the GLUT2 glucose transporter.
Clinical Use
The glucopyranose moiety of streptozocin confers both islet cell specificity and high water solubility to this nitrosourea-based antineoplastic. As a result, it is used exclusively in metastatic islet cell carcinoma of the pancreas and is administered IV in D5W or normal saline.
Side Effects
Lacking the 2-chloroethyl substituent of carmustine and lomustine, it is much less reactive as a DNA alkylating agent, and myelotoxicity is relatively rare but not unknown. Cumulative, dose-related renal toxicity can be severe or fatal, however, and 67% of patients receiving this drug will exhibit some kidney-related pathology.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplas tigenic, tumorigenic, and teratogenic data. Experimental poison by intravenous, parenteral, subcutaneous, and intraperitoneal routes. Moderately toxic to humans by intravenous route. Human systemic effects: nausea or vomiting, impaired liver function, kidney changes. Human mutation data reported. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx. See also NITROSAMINES.
Veterinary Drugs and Treatments
At present the primary purpose for streptozocin use in veterinary medicine is as a treatment for insulinomas in dogs, particularly those with refractory hypoglycemia and when tumors are nonresectable or have metastasized. Streptozocin potentially could be used for other oncologic conditions as well.
Carcinogenicity
Streptozotocin is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
STZ is an odorless ivory-colored crystalline powder or pale yellow crystals. Having the solubility of 5070 mg l-1 in water at 25 °C, STZ is soluble in alcohol and ketones and slightly soluble in polar organic solvents and insoluble in nonpolar organic solvents. STZ is produced by the soil microorganism and therefore isolated from soil samples that assumed a source of release to the environment. Vapor pressure and Henry’s law constant of STZ are 1.74E-12 mm Hg and 1.08E-10 cm3 per molecule-sec at 25 °C, respectively. No information is currently available for partition behavior in water, sediment, and soil; environmental persistency, long-range transport, or bioaccumulation/ biomagnification.
storage
-20°C
Purification Methods
Recrystallise streptozotocin from 95% EtOH. It is soluble in H2O, MeOH and Me2CO. It has UV max at 228nm ( 6360) in EtOH. The tetraacetate has m 111-114o(dec), and [] D 25 +41o (c 0.78, 95% EtOH) after recrystallisation from EtOAc. [Herr et al. J Am Chem Soc 89 4808 1967, NMR: Wiley et al. J Org Chem 44 9 1979.] It is a potent methylating agent for DNA [Bennett & Pegg Cancer Res 41 2786 1981].
Toxicity evaluation
STZ as an antibiotic is effective against Gram-negative bacteria. As a diabetogenic agent, STZ by its N-nitroso group acts as a nitric oxide donor in pancreatic islets. STZ inhibits synthesis of DNA in microorganisms and mammalian cells by alkylation and cross-linking the strands of DNA, and also affects on all stages of mammalian cell cycle. STZ-induced DNA damage causes activation of poly ADP-ribosylation, which is important in induction of diabetes rather than DNA damage. Biochemical studies indicated that STZ inhibits pyridine nucleotides and the key enzymes that are involved in glyconeogenesis. STZ transports glucose into the cell by the glucose transport protein GLUT2, but is not recognized by the other glucose transporters. Since beta cells have relatively high levels of GLUT2, this explains STZ’s relative toxicity in these cells.
References
1) Szkudelski?et al. (2001),?The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas.; Physiol. Res.,?50?537 2) Bennett & Pegg (1981),?Alkylation of DNA in rat tissues following administration of streptozotocin; Cancer Res.,?41?27861 3) Konrad?et al.?(2001),?The potential mechanism of the diabetogenic action of streptozotocin inhibition of pancreatic beta-cell O-GlcNAc-selective N-acetyl-beta-D-glucosaminidase; Biochem. J.,?356 Pt 1?31 4) Gao?et al.?(2000),?Streptozotocin-induced beta-cell death is independent of its inhibition of O-GlcNAcase in pancreatic Min6 cells; Arch. Biochem. Biophys.,?383?296 5) Kroncke & Kolb-Bachofen (1996),?Streptozotocin is not a spontaneous NO donor.; Free Radic. Res.,?24?77
Properties of Streptozotocin
| Melting point: | 121 °C (dec.) (lit.) |
| Boiling point: | 408.44°C (rough estimate) |
| alpha | D25 +39° |
| Density | 1.4410 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Water (up to 25 mg/ml) |
| pka | pKa 1.3 (Uncertain) |
| form | powder |
| color | white to light yellow |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 13,8912 |
| BRN | 2060675 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 18883-66-4 |
| IARC | 2B (Vol. 17, Sup 7) 1987 |
| EPA Substance Registry System | Streptozotocin (18883-66-4) |
Safety information for Streptozotocin
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H228:Flammable solids H341:Germ cell mutagenicity H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Streptozotocin
| InChIKey | ZSJLQEPLLKMAKR-FEQHFJGESA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Streptozotocin (STZ) for cell culture CAS 18883-66-4View Details
Streptozotocin (STZ) for cell culture CAS 18883-66-4View Details
18883-66-4 -
 Streptozotocin CAS 18883-66-4View Details
Streptozotocin CAS 18883-66-4View Details
18883-66-4 -
 STREPTOZOTOCIN 98% CAS 18883-66-4View Details
STREPTOZOTOCIN 98% CAS 18883-66-4View Details
18883-66-4 -
 Streptozocin CAS 18883-66-4View Details
Streptozocin CAS 18883-66-4View Details
18883-66-4 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
