Spermidine
Synonym(s):N-(3-Aminopropyl)-1,4-diaminobutane;1,8-Diamino-4-azaoctane;Additive Screening Solution 30/Kit-No 78374;Spermidine solution
- CAS NO.:124-20-9
- Empirical Formula: C7H19N3
- Molecular Weight: 145.25
- MDL number: MFCD00008229
- EINECS: 204-689-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
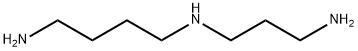
What is Spermidine?
Description
Spermidine is an endogenous polyamine. It is formed from putrescine by spermidine synthase.
Description
The tetraamine spermine is biosynthesized from the triamine spermidine, which is produced from the diamine putrescine. Anton van Leeuwenhoek, the microscopy pioneer, described spermine crystals in human semen in 1678, and spermidine was also first detected in sperm. Despite their names, both polyamines are ubiquitous in organisms and have several biological functions.
Chemical properties
Spermidine solution is a colorless clear liquid with very hygroscopic and oxidizable. It is soluble in water (50 mg/ml), ethanol, and ether.
Source
Spermidine is found in fresh green pepper, wheat germ, cauliflower, broccoli, mushrooms, and a variety of cheeses. Even higher amounts are found in soybean products such as natto, shitake mushrooms, amaranth grain and durian. Some of these ingredients are particularly common in a Mediterranean diet, which may explain why some believe eating like the Spanish can prolong your life.
The Uses of Spermidine
Spermidine can be used in electroporation while transferring the DNA into the cell under the electrical impulse. May be used for purification of DNA-binding proteins. Spermidine is also used, along with calcium chloride, for precipitating DNA onto microprojectiles for bombardment with a gene gun.
What are the applications of Application
Spermidine is a NOS1 inhibitor and NMDA and T4 polynucleotide kinase activator
Definition
ChEBI: Spermidine is a triamine that is the 1,5,10-triaza derivative of decane. It is a polyamine that is routinely included in restriction enzyme digestions to improve the cleavage efficacy of the DNA. Spermidine counteracts the inhibitory effects of contaminants coisolated with DNA and consequently permits complete digestion of the DNA at lower enzyme concentrations.
What are the applications of Application
Spermidine serves as a precursor of spermine and essential for both normal and neoplastic tissue growth. It was first detected in human sperm, but occurs widely in nature. It is essential in both normal and neoplastic tissue growth. Spermidine has a role in cell growth processes and the formation and interconversion of spermidine in mammalian cells has been reported. It has been studied in the regulation of tRNA methyltransferase activity and stimulates T4 polynucleotide kinase activity.
Preparation
The linker resin 2 was used in the synthesis of spermidine. In order to initiate the preparation the 2-nitrossulfonamide was anchored on the resin 2 by reaction in THF under reflux. The resulting resin 3 then reacted with 4-bromobutylphthalimide in acetonitrile in the presence of Cs2CO3 as base, generating the resin 4. Protected spermidine 5 was cleaved from resin 4 after treatment with hydrate hydrazine at room temperature. Spermidine 5 was previously prepared by our group in solution system utilizing Fukuyama's sulfonamide.
Synthesis and Characterization of a Linker for Primary Amines used in the Solid Phase Organic Synthesis of Spermidine
J. Braz. Chem. Soc., Vol. 22, No. 1, 86-91, 2011
DOI: 10.1590/S0103-50532011000100011
Origin
Spermidine was first discovered in 1678 by Dutch scientist Anton Van Leeuwenhoek in a sample of human semen. Shortly after, spermidine was discovered in human sperm. In the body, spermidine is created from its precursor putrescine.
Benefits
The biggest benefit of spermidine is its ability to reduce age-related diseases. It benefits cardioactive, spermidine can reduce the development of atherosclerosis in mice by reducing inflammation and improving cellular (mitochondria) function. It can reduce the formation of blood clots (platelet aggregation) and improve the normal dilation of cells lining blood vessels. Spermidine lowers blood pressure, improves energy use, and reduces heart cell death. It prevents neurologic diseases, and spermidine helps reduce cognitive, memory, and functional impairment associated with aging. It can also Decrease blood sugar levels, Increase autophagy, Increase bone strength, and have Anti-inflammatory effects.
Side Effects
The side effects of Spermidine included decreased levels of creatinine, calcium, and phosphate in the blood. These are markers of kidney function and electrolyte balance in the body, and if they are altered, it could signify a health problem. In particular, Spermidine supplements have not been tested in pregnant or nursing women. Another large observational study was done in humans that showed a link between higher blood levels of spermidine and an increased risk of stroke. Many supplemental forms of spermidine are derived from wheat germ. As a result, there may be trace levels of gluten in these supplements. For people with celiac disease, wheat allergy, or gluten sensitivity, it's important to check labels and choose a gluten-free supplement.
Mode of action
Spermidine's main mechanism of action is its ability to induce autophagy, a self-preservation process which clears out toxic, damaged and dysfunctional cells, resulting in lower levels of inflammation in the body. Autophagy is the main mechanism of spermidine in delaying aging and prolonging the lifespan. In addition, spermidine exerts its effects through other mechanisms, including anti-inflammation, histone acetylation reduction, lipid metabolism and regulation of cell growth and signaling pathways.
References
Madeo, F., Eisenberg, T., Pietrocola, F., et al. Spermidine in health and disease. Science 359(6374), eaan2788 (2018).
Lopatin, A.N., Makhina, E.N., and Nichols, C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372(6504), 366-369 (1994).
Eisenberg, T., Knauer, H., Schauer, A., et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11(11), 1305-1314 (2009).
Guo, X., Harada, C., Namekata, K., et al. Spermidine alleviates severity of murine experimental autoimmune encephalomyelitis. Invest. Ophthalmol. Vis. Sci. 52(5), 2696-2703 (2011).
Eisenberg, T., Abdellatif, M., Schroeder, S., et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22(12), 1428-1438 (2016).
Properties of Spermidine
| Melting point: | 23-25 °C |
| Boiling point: | 128-130 °C (14 mmHg) |
| Density | 1.00 g/mL at 20 °C |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| pka | 10.53±0.19(Predicted) |
| form | Liquid After Melting |
| Specific Gravity | 0.925 |
| color | Clear colorless |
| Odor | Ichtyal, ammoniacal |
| PH | 12.0-13.5 (25℃, 1M in H2O) |
| Water Solubility | Miscible with water, ethanol and ether. |
| Sensitive | Air Sensitive |
| λmax | λ: 260 nm Amax: 0.1 λ: 280 nm Amax: 0.05 |
| Merck | 14,8742 |
| BRN | 1698591 |
| Stability: | Stable, but air-sensitive and very hygroscopic - store under argon. Incompatible with acids, acid chlorides, acid anhydrides, oxidizing agents. |
| CAS DataBase Reference | 124-20-9(CAS DataBase Reference) |
| EPA Substance Registry System | 1,4-Butanediamine, N-(3-aminopropyl)- (124-20-9) |
Safety information for Spermidine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Spermidine
| InChIKey | ATHGHQPFGPMSJY-UHFFFAOYSA-N |
Spermidine manufacturer
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
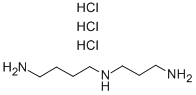
![Spermidine-[D8].3HCl](https://img.chemicalbook.in/CAS/20180703/GIF/1173019-26-5.gif)


You may like
-
 Spermidine, 99% CAS 124-20-9View Details
Spermidine, 99% CAS 124-20-9View Details
124-20-9 -
 Spermidine 95% CAS 124-20-9View Details
Spermidine 95% CAS 124-20-9View Details
124-20-9 -
 Spermidine 0.1 M solution CAS 124-20-9View Details
Spermidine 0.1 M solution CAS 124-20-9View Details
124-20-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1