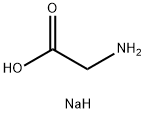
Sodium glycinate
- CAS NO.:6000-44-8
- Empirical Formula: C2H6NNaO2
- Molecular Weight: 99.06
- MDL number: MFCD00062543
- EINECS: 227-842-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:32
What is Sodium glycinate?
Description
Sodium glycinate is a non-essential amino acid. It is found primarily in gelatin and silk fibroin and used therapeutically as a nutrient. It is also a fast inhibitory neurotransmitter.
Chemical properties
White or light yellow crystalline powder. Hygroscopic, easily soluble in water.
The Uses of Sodium glycinate
Sodum glycinate is a useful research chemical. It Inhibits spinal cord neurotransmitter, allosteric regulator of NMDA receptors.
What are the applications of Application
Glycine sodium salt anhydrous is An inhibitory neurotransmitter
What are the applications of Application
Sodium glycinate is a substance with low toxicity, cost, and aggressiveness, and it is an effective cleaning option. Glycinate can react with silver compounds to form the polymeric compound silver glycinate (I), soluble in water. Glycinate also reacts with copper to form copper (II) glycinate, also soluble in water. Water solubility is due to the complexes’ ability to form hydrogen bonds. In addition to water solubility, which fulfills the criterion of keeping the cleaning reaction products within the solution, amino acids are capable of solubilizing silver and copper. Corrosion products can be removed using 0.1-mol L?1 sodium glycinate solution (pH 10) from artificially tarnished silver coins and naturally tarnished silver objects with a high silver content (90% or above)[1]. In the past sodium glycinate (SG) in glycerol was used in an immobilized liquid membrane in a closed loop life support systems, such as in spacecraft or space suits for removal of carbon dioxide from the atmosphere[2].
Hazard
Sodium glycinate is a compound that can be obtained by reacting the amino acid glycine with sodium hydroxide. Because glycinate is soluble in water, it requires less water for cleaning, and it is not a toxic substance or mixture, according to the GHS (Thermo Fisher Scientific, Safety Data Sheet). However, as the pH of the medium makes it reactive with skin, handling the sodium glycinate solution requires gloves. Another difference between glycinate and thiourea is that the former does not cause wear on the metallic surface. Thiourea, however, causes micro-roughness. Thus, polishing can be required after the treatment.
References
[1] Ho-Jun Song. “Solubilities of carbon dioxide in aqueous solutions of sodium glycinate.” Fluid Phase Equilibria 246 1 (2006): Pages 1-5.
[2] Jo?o Cura D’Ars de Figueiredo Junior. “The Cleaning of Silver Objects With a Basic Solution of Sodium Glycinate: A Study on Artificially and Naturally Tarnished Silver.” Studies in Conservation 66 1 (2021): 375–383.
Properties of Sodium glycinate
| Boiling point: | 321℃[at 101 325 Pa] |
| Density | 1.333[at 20℃] |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Moist Beads |
| pka | 4.3[at 20 ℃] |
| color | White to yellow |
| InChI | InChI=1S/C2H5NO2.Na.H/c3-1-2(4)5;;/h1,3H2,(H,4,5);; |
| CAS DataBase Reference | 6000-44-8(CAS DataBase Reference) |
| EPA Substance Registry System | Glycine, monosodium salt (6000-44-8) |
Safety information for Sodium glycinate
Computed Descriptors for Sodium glycinate
| InChIKey | MFPUSDPJVVFIGR-UHFFFAOYSA-N |
| SMILES | C(=O)(O)CN.[NaH] |
Sodium glycinate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 6000-44-8 Glycine monosodium 98%View Details
6000-44-8 Glycine monosodium 98%View Details
6000-44-8 -
 Sodium glycinate 98% CAS 6000-44-8View Details
Sodium glycinate 98% CAS 6000-44-8View Details
6000-44-8 -
 Sodium GlycinateView Details
Sodium GlycinateView Details
6000-44-8 -
 Industrial Grade Sodium Glycinate (6000-44-8), Packaging Type: Hdpe Bag, 25 kgView Details
Industrial Grade Sodium Glycinate (6000-44-8), Packaging Type: Hdpe Bag, 25 kgView Details
6000-44-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
