SIMVASTATIN, SODIUM SALT
- CAS NO.:101314-97-0
- Empirical Formula: C25H41NaO6
- Molecular Weight: 460.59
- MDL number: MFCD04974206
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43

What is SIMVASTATIN, SODIUM SALT?
Description
Simvastatin is a competitive inhibitor of HMG-CoA reductase (Ki = 0.12 nM). Simvastatin reduces plasma cholesterol levels in rats and dogs when administered at doses of 1.2 and 8 mg/kg, respectively. Simvastatin suppresses TNF-induced NF-κB activation (IC50 = ~13 μM) and potentiates apoptosis in human myeloid leukemia cells. It also inhibits glutathione peroxidase 4 (GPX4) activity, increases malondialdehyde (MDA) levels, and induces ferroptosis in MDA-MB-231 and MCF-7 breast cancer cells. Formulations containing simvastatin have been used in the treatment of dyslipidemias.
The Uses of SIMVASTATIN, SODIUM SALT
Simvastatin Hydroxy Acid Sodium Salt is a metabolite of Simvastatin (S485000), a synthetic derivative of a fermentation product of Aspergillus terreus. A competitive inhibitor of HMG-CoA reductase. A synthetic analog of Lovastatin. Antilipemic. Simvastatin, the drug, is sold under the trade name Zocor.
What are the applications of Application
Simvastatin Hydroxy Acid Sodium Salt is Simvastatin Hydroxy Acid Sodium Salt is an inhibitor of HMGCR.
What are the applications of Application
Simvastatin, Sodium Salt is an HMGCR inhibitor
in vitro
previous study found that simvastatin could inhibit the incorporation of 14c-acetate to 14c-sterol with an ic50 value of 15 nm in cultured hep g2 cells. in addition, simvastatin was found to be a potent inhibitor of cholesterol synthesis in cultured liver cells, whereas pravastatin inhibited cholesterol synthesis in liver cells only after these cells had been digested by collagenase [1].
in vivo
animal study showd that rats orally dosed with simvastatin had lower plasma cholesterol levels after 4 days of treatment. at the level of 0.02% of the diet, simvastatin lowered plasma cholesterol levels in rats by 64%. moreove, in dogs, simvastatin at a daily oral dosage of 8 mg/kg lower levels of plasma cholesterol. at this dosage, simvastatin was slightly more potent than lovastatin and the levels of plasma cholesterol in these dogs returned to pretreatment levels after stopping the treatment [1].
References
[1] chao, y. ,chen, j.s.,hunt, v.m., et al. lowering of plasma cholesterol levels in animals by lovastatin and simvastatin. european journal of clinical pharmacology 40, s11-s14 (1991).
[2] s martande s, kumari m, pradeep ar, pal singh s, kumar suke d. ncomparative evaluation of efficacy of subgingivally delivered 1.2% atorvastatin and 1.2% simvastatin in the treatment of intrabony defects in chronic periodontitis: a randomized controlled trial. j dent res dent clin dent prospects. 2017 winter;11(1):18-25.
Properties of SIMVASTATIN, SODIUM SALT
| Melting point: | >122°C (dec.) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | White powder solid. |
| color | White to Pale Beige |
| Stability: | Hygroscopic |
Safety information for SIMVASTATIN, SODIUM SALT
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for SIMVASTATIN, SODIUM SALT
SIMVASTATIN, SODIUM SALT manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
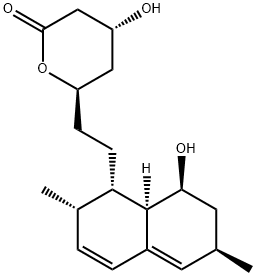
 101314-97-0 98%View Details
101314-97-0 98%View Details
101314-97-0 -
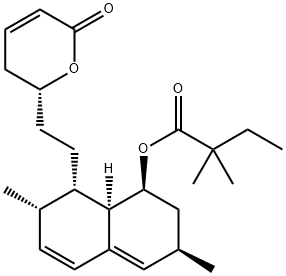
 Simvastatin Sodium 98%View Details
Simvastatin Sodium 98%View Details
101314-97-0 -
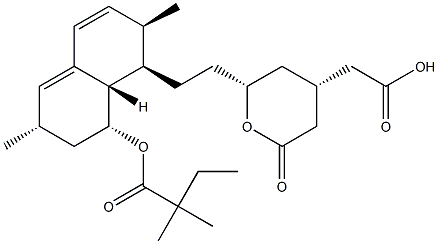
 Simvastatin Sodium 101314-97-0 98%View Details
Simvastatin Sodium 101314-97-0 98%View Details
101314-97-0 -
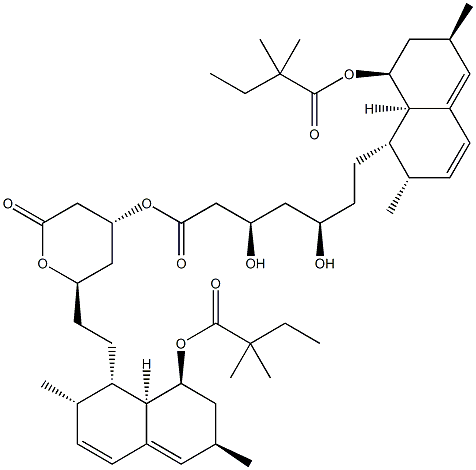
 Simvastatin EP Impurity A 95.88View Details
Simvastatin EP Impurity A 95.88View Details
101314-97-0 -
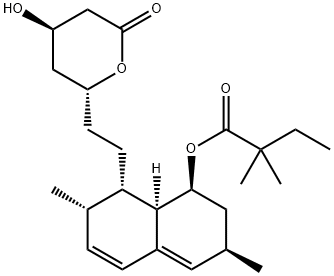
 Simvastatin, Sodium Salt CASView Details
Simvastatin, Sodium Salt CASView Details -
 SIMVASTATIN EP IMPURITY A 90 % AboveView Details
SIMVASTATIN EP IMPURITY A 90 % AboveView Details
101314-97-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
