SILICATE
- Empirical Formula: O3Si-2
- Molecular Weight: 76.0837
- Update Date: 2023-02-15 16:18:19
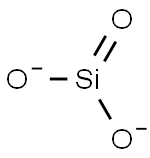
What is SILICATE?
The Uses of SILICATE
Fillers in plastics and rubber, paper coatings, antacids, anticaking agents, cements.
Definition
Any of the widely occurring compounds containing silicon, oxygen, and one or more metals with or without hydrogen. The silicon and oxygen may combine with organic groups to form silicate esters. Most rocks (except limestone and dolomite) and many mineral
Definition
silicate: Any of a group of substancescontaining negative ionscomposed of silicon and oxygen. Thesilicates are a very extensive groupand natural silicates form the majorcomponent of most rocks (see silicateminerals). The basic structural unit is the tetrahedral SiO4 group. This may occur as a simple discrete SiO44– anion as in the orthosilicates, e.g. phenacite (Be2SiO4) and willemite (Zn2SiO4). Many larger silicate species are also found (see illustration). These are composed of SiO4 tetrahedra linked by sharing oxygen atoms as in the pyrosilicates, Si2O76–, e.g. Sc2Si2O7. The linking can extend to such forms as bentonite, BaTiSi3O9, or alternatively inÜnite chain anions, which are single strand (pyroxenes) or double strand (amphiboles). Spodumene, LiAl(SiO3)2, is a pyroxene and the asbestos minerals are amphiboles. Large two-dimensional sheets are also possible, as in the various micas (see illustration), and the linking can extend to full threedimensional framework structures, often with substituted trivalent atoms in the lattice. The zeolites are examples of this.
Hazard
(Natural silicate dusts) Toxic by inhalation.
Agricultural Uses
Any of the widely occurring compounds containing
silicon, oxygen and one or more metals with or without
hydrogen is called silicate. The silicon and oxygen may
combine with organic groups to form similar esters. Most
rocks and many minerals, except limestone and dolomite,
are silicates. Typical natural silicates are gemstones,
except for diamond. Portland cement, because it is
manufactured from chalk and clay, contains a high
percentage of calcium silicates.
Sodium silicate is the best known of the synthetic
silicates.
Safety information for SILICATE
New Products
1-Amino-1-cyclohexanecarboxylic acid 2-Hydroxyacetamide Ethyl 5-cyclopropyl-1H-pyrazole-3-carboxylate 2,4,6-Trichloroaniline ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole (4-(4-Fluorophenoxy)phenyl)methanamine Tert-butyl N-(pent-4-yn-2-yl) carbamate 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine 2-chloro-4-methyl-5-nitro pyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA]You may like
-
 609-15-4 Ethyl-2-ChloroacetoacetateView Details
609-15-4 Ethyl-2-ChloroacetoacetateView Details
609-15-4 -
 221615-75-4 99%View Details
221615-75-4 99%View Details
221615-75-4 -
 609-15-4 Ethyl-2-ChloroacetoacetateView Details
609-15-4 Ethyl-2-ChloroacetoacetateView Details
609-15-4 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
![2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 2033-24-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 2033-24-1 98%View Details
2,2-Dimethyl-1,3-Dioxane-4,6-Dione [Meldrum Acid] 2033-24-1 98%View Details
2033-24-1 -
 (Z)-Ethyl 2-chloro-2-(2-(4-methoxyphenyl)hydrazono) acetateView Details
(Z)-Ethyl 2-chloro-2-(2-(4-methoxyphenyl)hydrazono) acetateView Details
27143-07-3 -
 CIS- BROMO BENZOATE 61397-56-6View Details
CIS- BROMO BENZOATE 61397-56-6View Details
61397-56-6