Salicylamide
Synonym(s):2-Hydroxybenzamide;Salicylamide
- CAS NO.:65-45-2
- Empirical Formula: C7H7NO2
- Molecular Weight: 137.14
- MDL number: MFCD00007978
- EINECS: 200-609-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
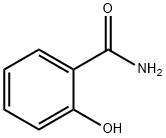
What is Salicylamide?
Toxicity
Oral, rat LD50: 1890 mg/kg
Description
Salicylamide is less acidic (pKa 8.2) than other salicylic acid derivatives. Although poorly soluble in water, stable solutions can be formed at pH 9 through ionization of the phenolic group. It is absorbed from the GI tract on oral administration and is rapidly metabolized to inactive metabolites by intestinal mucosa, but not by hydrolysis. Activity appears to reside in the intact molecule. Salicylamide is approximately 40 to 55% plasma protein bound, and it competes with other salicylates and acetaminophen for glucuronide conjugation, decreasing the extent of conjugation of these other drugs.
Chemical properties
white or light pink crystals or powder
The Uses of Salicylamide
salicylamide is an analgesic, fungicide, and anti-inflammatory ingredient used to soothe the skin. Salicylamide is an aromatic amide.
The Uses of Salicylamide
Medicine (analgesic).
The Uses of Salicylamide
Salicylamide is used in combination with both aspirin and caffeine in the over-the-counter pain remedies. It is used as an analgesic and as an antipyretic.
What are the applications of Application
Salicylamide is an analgesic and antipyretic
Background
Salicylamide is the common name for the substance o-hydroxybenzamide, or amide of salicyl. Salicylamide is a non-prescription drug with analgesic and antipyretic properties. It has similar medicinal uses to aspirin. Salicylamide is used in combination with both aspirin and caffeine in the over-the-counter pain remedies
Definition
ChEBI: The simplest member of the class of salicylamides derived from salicylic acid.
General Description
Odorless white or slightly pink crystals. Bitter taste, leaves a sensation of warmth on the tongue. pH (saturated aqueous solution at 82°F) about 5. Sublimation begins at the melting point.
General Description
Salicylamide, o-hydroxybenzamide, is a derivative of salicylicacid that is fairly stable to heat, light, and moisture. Itreportedly exerts a moderately quicker and deeper analgesiceffect than aspirin because of quicker CNS penetration. Its metabolismdiffers from aspirin, because it is not metabolized tosalicylic acid but rather excreted exclusively as the ether glucuronideor sulfate. Thus, as a result of lack of contributionfrom salicylic acid, it has a lower analgesic and antipyretic efficacythan that of aspirin. However, it can be used in place ofsalicylates for patients with a demonstrated sensitivity to salicylates.It is also excreted much more rapidly than other salicylates,which accounts for its lower toxicity. It is available inseveral nonprescription products, in combination with acetaminophenand phenyltoloxamine (e.g., Rid-A Pain compound,Cetazone T, Dolorex, Ed-Flex, Lobac) or with aspirin,acetaminophen, and caffeine (e.g., Saleto, BC Powder).
Air & Water Reactions
Salicylamide darkens on exposure to air. . Insoluble in water.
Reactivity Profile
Salicylamide is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Salicylamide may be sensitive to prolonged exposure to light.
Fire Hazard
Flash point data for Salicylamide are not available; however, Salicylamide is probably combustible.
Clinical Use
Whereas salicylamide is reported to be as effective as aspirin as an analgetic/antipyretic and is effective in relieving pain associated with arthritic conditions, it does not appear to possess useful anti-inflammatory activity. Thus, indications for the treatment of arthritic disease states are unwarranted, and its use is restricted to the relief of minor aches and pain at a dosage of 325 to 650 mg three or four times per day. Its effects in humans are not reliable, however, and its use is not widely recommended.
Metabolism
Not Available
Purification Methods
Crystallise the amide from water or repeatedly from CHCl3 [Nishiya et al. J Am Chem Soc 108 3880 1986]. [Beilstein 10 IV 169.] The anilide [87-17-2] M 213.2, m 135o crystallises from H2O. [Beilstein 12 H 500, 12 I 268, 12 II 256, 12 944.]
Properties of Salicylamide
| Melting point: | 140-144 °C(lit.) |
| Boiling point: | 270°C |
| Density | 1,175 g/cm3 |
| refractive index | 1.5323 (estimate) |
| Flash point: | 181°C/14mm |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | methanol: 0.1 g/mL, clear |
| form | Crystalline Powder |
| pka | pKa 8.13(H2O
t = 37) (Uncertain) |
| color | White |
| Odor | Odorless |
| PH Range | 5 (0.2% aq soln) |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
| Decomposition | 270°C |
| Merck | 14,8328 |
| BRN | 742439 |
| Stability: | Stable. Light sensitive. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 65-45-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzamide, 2-hydroxy-(65-45-2) |
| EPA Substance Registry System | o-Hydroxybenzamide (65-45-2) |
Safety information for Salicylamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Salicylamide
| InChIKey | SKZKKFZAGNVIMN-UHFFFAOYSA-N |
Salicylamide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Salicylamide 99%View Details
Salicylamide 99%View Details
65-45-2 -
 Salicylamide 99%View Details
Salicylamide 99%View Details -
 Salicylamide pure CAS 65-45-2View Details
Salicylamide pure CAS 65-45-2View Details
65-45-2 -
 Salicylamide CAS 65-45-2View Details
Salicylamide CAS 65-45-2View Details
65-45-2 -
 Salicylamide CAS 65-45-2View Details
Salicylamide CAS 65-45-2View Details
65-45-2 -
 SALICYLAMIDE Extra Pure CAS 65-45-2View Details
SALICYLAMIDE Extra Pure CAS 65-45-2View Details
65-45-2 -
 Powder Deferasirox Benzamide Impurity, 65-45-2View Details
Powder Deferasirox Benzamide Impurity, 65-45-2View Details
65-45-2 -
 Salicylamide Cas 65 45 2, IPView Details
Salicylamide Cas 65 45 2, IPView Details
65-45-2
