(S)-4-Methyl-2,5-oxazolidonedione
- CAS NO.:2224-52-4
- Empirical Formula: C4H5NO3
- Molecular Weight: 115.09
- MDL number: MFCD03411276
- EINECS: 218-750-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-25 12:39:57
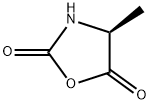
What is (S)-4-Methyl-2,5-oxazolidonedione?
Description
(S)-4-Methyl-2,5-oxazolidonedione is also known as N-Carboxy anhydrides. The Fuchs-Farthing method, reported in 1922, was the only practical method for preparing (S)-4-Methyl-2,5-oxazolidonedione[1]. N-Carboxy anhydrides (NCAs) are important as α-amino acid building blocks for the synthesis of oligopeptides and nonpeptide compounds, and for the synthesis of polypeptides[2]. The polymerization of NCAs is the main process for the preparation of polypeptides, and its purity and side chains can significantly affect the synthesized polypeptides. NCAs polymerizations have been initiated using many different nucleophiles and bases, the most common being primary amines and alkoxide anions[3].
The Uses of (S)-4-Methyl-2,5-oxazolidonedione
(S)-4-Methyl-2,5-oxazolidonedione is a reagent used in the synthesis of poly(γ-benzyl-L-glutamate) and its block polypeptides with alanine, leucine and phenylalanine.
The Uses of (S)-4-Methyl-2,5-oxazolidonedione
(S)-4-Methyl-2,5-oxazolidonedione is composed of oxazolidine ring and 2,5-dione ring. It exhibits chirality, meaning it exists as two non-superimposable mirror image forms. Therefore, it can be used as an organic reagent for the synthesis of enantiomeric pure substances. (S)-4-Methyl-2,5-oxazolidonedione is a reagent used in the synthesis of poly(γ-benzyl-L-glutamate) and its block polypeptides with alanine, leucine and phenylalanine.
Production Methods
L-alanine (20.0 g, 0.224 mol) and triphosgene (53.4 g, 0.180 mol) were suspended in 400.0 mL of dry THF bubbled with nitrogen flux in a flame-dried three-neck flask. The mixture was stirred at 60 °C for 2 h before further bubbling with nitrogen flux for 30 min. After that, the solution was precipitated in 1000.0 mL of n-hexane and stored at -20 °C. The supernatant was removed, and the residues were collected and dissolved in 200.0 mL of ethyl acetate, before two washings with 100.0mL of ice-cold water and one washing with 100.0 mL of 0.5% NaHCO3 ice-cold aqueous solution. The organic phase was then dried over anhydrous MgSO4 and evaporated to obtain 15.5 g of L-Ala NCA ((S)-4-Methyl-2,5-oxazolidonedione). The yield of L-Ala NCA was 77.5%.

Synthesis Reference(s)
The Journal of Organic Chemistry, 50, p. 2200, 1985 DOI: 10.1021/jo00212a042
References
[1] Otake Y, et al. Rapid and Mild Synthesis of Amino Acid N-Carboxy Anhydrides
Using Basic-to-Acidic Flash Switching in a Micro-flow Reactor. Angewandte Chemie International Edition, 2018; 57: 11389-11393.
[2] Sch?fer G, et al. Synthesis of Sterically Hindered N-Acylated Amino Acids from N-Carboxyanhydrides. Organic Letters, 2014; 16: 1526–1529.
[3] Imanishi Y, et al. Polymerization of α-amino acid N-carboxyanhydride in the presence of preformed poly(α-amino acid) - from chain effect to stereoselective polymerization. Pure and Applied Chemistry, 1981; 53.
Properties of (S)-4-Methyl-2,5-oxazolidonedione
| Melting point: | 92℃ |
| alpha | +5.13°(25/D,c=6.4,dioxane) |
| Density | 1.296 |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Very Slightly, Heated) |
| form | Solid |
| pka | 9.38±0.40(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C4H5NO3/c1-2-3(6)8-4(7)5-2/h2H,1H3,(H,5,7)/t2-/m0/s1 |
Safety information for (S)-4-Methyl-2,5-oxazolidonedione
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (S)-4-Methyl-2,5-oxazolidonedione
| InChIKey | DTETYCNJKAUROO-REOHCLBHSA-N |
| SMILES | O1C(=O)[C@H](C)NC1=O |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1