Romiplostim
- CAS NO.:267639-76-9
- Empirical Formula: C15H14N2O
- Molecular Weight: 238.28446
- Update Date: 2025-03-11 11:38:41
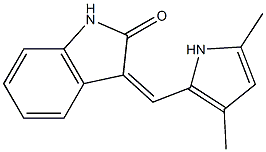
What is Romiplostim?
Description
Romiplostim has been developed and launched for the treatment of thrombocytopenia in patients with ITP, an autoimmune blood disorder in which there is autoantibody-mediated platelet destruction. Coating of the platelets by autoantibodies results in accelerated ingestion by macrophages. In addition to the destruction component, ITP is also characterized by impaired platelet production. The primary physiological regulator of platelet production is thrombopoietin (TPO), a growth factor that orchestrates its effects through the TPO (Mpl) receptor.Agonists of this receptor could, thereby, remediate the platelet production contribution of ITP. By binding to and activating the TPO receptor, romiplostim serves as a TPO receptor agonist. As a recombinant fusion protein, in this case coined as a peptibody, it was engineered to contain two identical single-chain subunits, each consisting of a TPO-binding domain linked to the C-terminus of a human IgG1 Fc domain designed to increase the half-life of the protein. Although it binds to the TPO receptor, romiplostim has no sequence homology to endogenous TPO, which should mitigate the risks encountered with the first generation predecessor, recombinant megakaryocyte growth and development factor (MGDF); this truncated, non-glycosylated form of TPO conjugated to PEG was halted during clinical studies because of the immunogenicity of PEG-MGDF resulting in cross-reacting (neutralizing) antibodies against endogenous TPO and subsequent severe, unrelenting thrombocytopenia. Romiplostim also avoids the side effects associated with the generalized immunosuppressive agents previously employed for the treatment of ITP and may prevent splenectomy, another common treatment option.
Originator
Amgen (United States)
brand name
Nplate
Clinical Use
Fc-peptide fusion protein:
Treatment of chronic immune (idiopathic)
thrombocytopenic purpura (ITP)
Metabolism
Mainly renal clearance.
Safety information for Romiplostim
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidYou may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
