Ristocetin A sulfate
Synonym(s):Ristocetin sulfate salt
- CAS NO.:11140-99-1
- Empirical Formula: C95H112N8O48S
- Molecular Weight: 2166
- MDL number: MFCD00076121
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-17 18:22:24
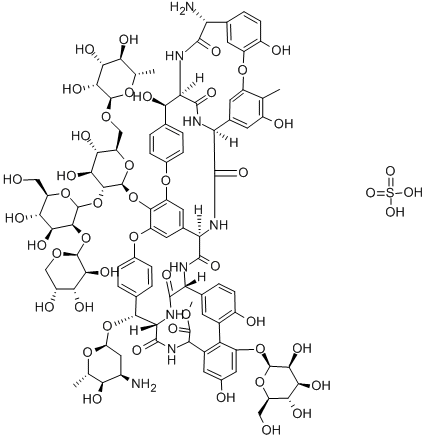
What is Ristocetin A sulfate?
The Uses of Ristocetin A sulfate
Ristocetin A sulfate is a potent antibacterial glycopeptide antibiotic that was withdrawn from clinical use following a high incidence of thrombocytopenia. Further investigation found that ristocetin A induced platelet aggregation by binding to a factor absent in people suffering from von Willebrand's disease, leading to the use of ristocetin A as an important diagnostic aid.
The Uses of Ristocetin A sulfate
Ristomycin Monosulfate is used in biological studies for ligand discovery of fluorescent HIV-1 Dimerization Initation Site.
What are the applications of Application
Ristocetin A sulfate is an antibiotic with platelet aggregation activity
General Description
Ristomycin/ristocetin is a type III glycopeptide. It is a highly glycosylated glycopeptide, previously found in?Amycolatopsis lurida.
Biological Activity
ristocetin a, a glycopeptide related to vancomycin, is an antibiotic produced by the microorganism nocardia lurida[1].ristocetin a is currently in clinical use to treat bacterial infections [1]. ristocetin a is an antibiotic which can be used to treat staphylococcal infections. the side effects of ristocetin a include thrombocytopenia and platelet agglutination. ristocetin a has been used in two assays: the ristocetin cofactor assay and the ristocetin-induced platelet aggregation assay. these two assays could be used to diagnosis the von willebrand disease and other bleeding disorders [2, 3]. the structural features of ristocetin a are similar to vancomycin.
Biochem/physiol Actions
Ristocetin activates platelets. Ristocetin-induced platelet agglutination (RIPA) is decreased in patients undergoing chronic hemodialysis. Together with increased plasma glycocalicin levels (a product of enzymatic cleavage of GPIb), RIPA may contribute to diminished platelet adhesion to vascular subendothelium and increased bleeding associated with uremia. Addition of ristocetin and vWf caused specific agglutination of rGPIbα-liposomes suporting the potential use of rGPIbα-liposomes to enhance platelet function in vivo and support hemostasis in thrombocytopenic individuals.
References
[1] pearson a j, heo j n. approaches to the fully functionalized def ring system of ristocetin a via highly selective ruthenium-promoted snar reaction[j]. organic letters, 2000, 2(19): 2987-2990.hawks g h. antibiotic therapy of staphylococcal infections[j]. canadian medical association journal, 1965, 93(16): 848.
[3] castaman, g. ,hillarp, a. and goodeve, a. laboratory aspects of von willebrand disease: test repertoire and options for activity assays and genetic analysis. haemophilia 20(suppl 4), 65-70 (2014).
Properties of Ristocetin A sulfate
| Melting point: | >200°C (dec.) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Water (Slightly) |
| form | Light tan solid. |
| color | White to Pale Beige |
| Stability: | Hygroscopic |
Safety information for Ristocetin A sulfate
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Ristocetin A sulfate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![N-[2-(4-methoxyphenyl)ethyl]-2-(methylamino)acetamide](https://img.chemicalbook.in/CAS/GIF/77335-15-0.gif)
![[1-(4-ETHOXY-PHENYL)-ETHYL]-METHYL-AMINE](https://img.chemicalbook.in/CAS/GIF/842955-24-2.gif)

You may like
-
 Ristomycin monosulfate CAS 11140-99-1View Details
Ristomycin monosulfate CAS 11140-99-1View Details
11140-99-1 -
 DANISH TYPE FLOUR MILL RISTON BRAND SPECIFICALLY MADE FOR AFRICA AND MIDDLE EAST MARKET.View Details
DANISH TYPE FLOUR MILL RISTON BRAND SPECIFICALLY MADE FOR AFRICA AND MIDDLE EAST MARKET.View Details
11140-99-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
