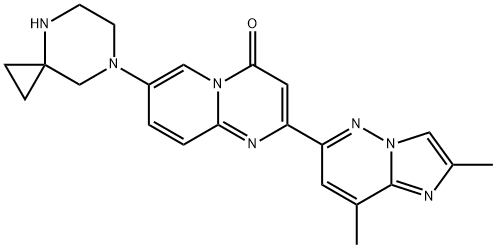
Risdiplam
- CAS NO.:1825352-65-5
- Empirical Formula: C22H23N7O
- Molecular Weight: 401.46
- MDL number: MFCD31657372
- Update Date: 2024-11-19 15:53:33
What is Risdiplam?
Absorption
The Tmax following oral administration is approximately 1-4 hours. Following once-daily administration with a morning meal (or after breastfeeding), risdiplam reaches steady-state in approximately 7-14 days. The pharmacokinetics of risdiplam were found to be approximately linear between all studied dosages in patients with SMA.
Toxicity
Data regarding overdose of risdiplam are unavailable. Symptoms of overdose are likely to be consistent with risdiplam's adverse effect profile, and may therefore involve significant fever, diarrhea, and skin reactions.
Description
Risdiplam is a survival of motor neuron 2 (SMN2) splicing modifier.
Chemical properties
light yellow powder to yellow powder
The Uses of Risdiplam
Risdiplam is used in preparation of Imidazopyridazinyl Pyridopyrimidinones for treating spinal muscular atrophy. Gene splicing modulator.
Background
Risdiplam is an orally bioavailable mRNA splicing modifier used for the treatment of spinal muscular atrophy (SMA). It increases systemic SMN protein concentrations by improving the efficiency of SMN2 gene transcription. This mechanism of action is similar to its predecessor nusinersen, the biggest difference being their route of administration: nusinersen requires intrathecal administration, as does the one-time gene therapy onasemnogene abeparvovec, whereas risdiplam offers the ease of oral bioavailability.
Risdiplam was approved by the FDA in August 2020 for the treatment of spinal muscular atrophy (SMA). Set to be substantially cheaper than other available SMA therapies, risdiplam appears to provide a novel and relatively accessible treatment option for patients with SMA regardless of severity or type.
Indications
Risdiplam is indicated for the treatment of spinal muscular atrophy (SMA).
Pharmacokinetics
Risdiplam helps to alleviate symptoms of spinal muscular atrophy by stimulating the production of a critical protein in which these patients are deficient. Early trials with risdiplam demonstrated up to a 2-fold increase in SMN protein concentration in SMA patients after 12 weeks of therapy.
Side Effects
Body aches or pain, chest pain or tightness, chills, cough, ear congestion, fever, headache, loss of voice, runny or stuffy nose, sneezing, sore throat, trouble breathing, unusual tiredness or weakness and so on.
Metabolism
The metabolism of risdiplam is mediated primarily by flavin monooxygenases 1 and 3 (FMO1 and FMO3), with some involvement of CYP1A1, CYP2J2, CYP3A4, and CYP3A7. Parent drug comprises approximately 83% of circulating drug material.
A pharmacologically-inactive metabolite, M1, has been identified as the major circulating metabolite - this M1 metabolite has been observed in vitro to inhibit MATE1 and MATE2-K transporters, similar to the parent drug.
storage
0-4℃ for short term (days to weeks) or -20℃ for long term (months)
Properties of Risdiplam
| Density | 1.50±0.1 g/cm3(Predicted) |
| solubility | Soluble in DMSO |
| form | solid |
| pka | 9.41±0.20(Predicted) |
| color | Light yellow to yellow |
| InChI | InChI=1S/C22H23N7O/c1-14-9-18(26-29-11-15(2)24-21(14)29)17-10-20(30)28-12-16(3-4-19(28)25-17)27-8-7-23-22(13-27)5-6-22/h3-4,9-12,23H,5-8,13H2,1-2H3 |
Safety information for Risdiplam
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Risdiplam
| InChIKey | ASKZRYGFUPSJPN-UHFFFAOYSA-N |
| SMILES | C12C=CC(N3CC4(CC4)NCC3)=CN1C(=O)C=C(C1=NN3C=C(C)N=C3C(C)=C1)N=2 |
Risdiplam manufacturer
Biophore India Pharmaceuticals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateYou may like
-
 1825352-65-5 Risdiplam 98%View Details
1825352-65-5 Risdiplam 98%View Details
1825352-65-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1